Verification and Validation Testing of Medical Device Software
Be 100% Ready for Clinical Validation
In healthcare IT since 2005, ScienceSoft helps deliver patient-safe medical device software fully compliant with healthcare industry standards and regulations.
Medical device software verification and validation (V&V) testing ensures that software for medical devices and accessories meets its functional and non-functional requirements and fulfills the target users’ needs. It may include integration and regression testing, requirements-based system verification, performance testing, architecture validation, unit testing, code review, and more.
ScienceSoft is ready to provide comprehensive verification and validation testing to help you produce quality and safe medical device software meeting IEC 62304, FDA and other international healthcare regulations
Scope of V&V Testing by ScienceSoft
Companies we assist
- Medical device manufacturers.
- Medical device software providers.
Medical device software we test
We cover all 3 software safety classes as defined by IEC 62304 (requirements to medical device software lifecycle processes):
- Class A – software that cannot cause any injury or damage to health.
- Class B – software that can cause minor harm, such as non-serious injuries.
- Class C – the software that can cause major harm, such as severe injuries or death.
How We Handle the Testing Process
1.
Analysis and planning
We thoroughly analyze the functional and non-functional requirements of medical device software to outline the future verification and validation testing strategy. The amount and granularity of the required testing documentation will depend on the type of medical device software and its safety class.
ScienceSoft’s experienced QA managers create a verification and validation testing plan (together with our in-house healthcare consultants), choose the appropriate testing tools and assemble a project team. The team may consist of QA engineers, developers (e.g., for unit testing, static code analysis), security testers and a compliance consultant.
2.
Verification & validation testing
Our team conducts verification and validation testing to ensure your software is secure, regulatory-compliant and functions as intended. The scope of software validation and verification testing activities will depend on the software safety class.
Check the most common activities within V&V testing.
- Review of the software functional and non-functional requirements for clarity, consistency, and traceability.
- Checking software architecture and design for unambiguity, security, and compliance with software architecture and design requirements.
- Code review.
- Static code analysis to detect errors early before software is executed.
- Dynamic code analysis to catch the possible performance problems, memory issues, and software crashes during software execution.
- Unit testing. Required for software safety classes B and C.
- Integration testing. Required for software safety classes B and C.
- System testing, including manual or automated functional and non-functional testing (performance, usability, reliability, security testing). Required for software classes A, B and C.
- User acceptance testing.
3.
Reporting on V&V testing results
In compliance with IEC 62304, we provide detailed and coherent documentation related to verification and validation testing activities:
- A detailed software verification and validation testing plan with the defined acceptance criteria.
- Software requirements, architecture, and design verification reports.
- Software testing plan at the API, integration, and system levels.
- Manual test cases and automated test scripts.
- Test results reports: test execution reports, test summary reports, detailed defects descriptions with root cause analysis, and software performance reports.
Why Entrust Your Medical Device Software to ScienceSoft
- Since 1989 in software testing, since 2005 in healthcare IT services.
- A solid portfolio of 150+ successful projects for the healthcare IT domain.
- Doctor of Medicine consultant on board.
- Practical experience with HIPAA, GDPR, and Cures Act requirements, healthcare interoperability standards (HL7, FHIR, SNOMED CT, LOINC, RxNorm, XDS/XDS-I), and FDA/CE registration.
- Compliance with ISO 13485 to guarantee medical software testing according to the FDA and MDR requirements.
- ISO 27001-certified security management system to ensure full protection of the customers’ data entrusted to us.
- Mature quality management system confirmed by ISO 9001 certification.
Our awards, recognitions, and certifications
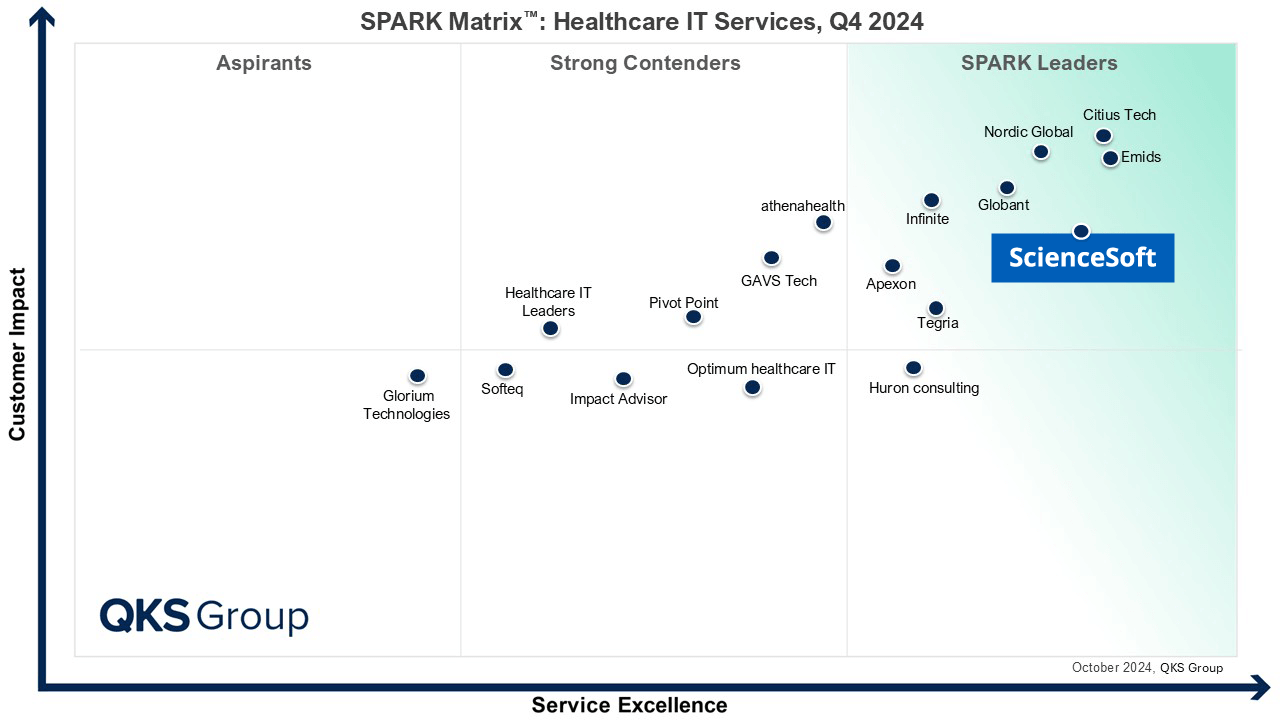
Featured among Healthcare IT Services Leaders in the 2022 and 2024 SPARK Matrix
Recognized for Healthcare Technology Leadership by Frost & Sullivan in 2023 and 2025
Named among America’s Fastest-Growing Companies by Financial Times, 4 years in a row

Top Healthcare IT Developer and Advisor by Black Book™ survey 2023
Four-time finalist across HTN Awards programs

Named to The Healthcare Technology Report’s Top 25 Healthcare Software Companies of 2025

HIMSS Gold member advancing digital healthcare
ISO 13485-certified quality management system
ISO 27001-certified security management system
Choose Your Service Option
Technologies We Use in Our V&V Testing Projects
Mobile application testing tools
Performance testing tools
API testing tools
Security testing tools
Automated UI testing tools
CI/CD tools
Test management and defect tracking software
Make the Most of Validation & Verification Testing with ScienceSoft
Our clients’ business interests are our primary concern. See how you can benefit from medical device software V&V testing with ScienceSoft:
-20-40% software testing costs due to KPIs-based testing, reusable test documentation and test cases, and optimal prioritization of testing activities.
Easier FDA/CE submission due to exhaustive testing, absence of health-hazard software errors, and comprehensive testing documentation according to ISO 13485 and ISO/IEC/IEEE 29119-3:2021.
Proven protection against data breaches due to professional security and compliance testing.