IoT for Connected Medical Devices
Use Cases, IoMT Architecture, and Tech Stack
With decades-long experience in healthcare IT and IoT, ScienceSoft designs and develops IoT systems connecting medical devices.
IoT for Connected Medical Devices In Brief
Used for diagnostics, care delivery, disease management and prevention, IoT for connected medical devices reduces unnecessary clinic visits by 25% and hospital stay by 33%, and saves up to 30% of medical staff time for vitals entry.
Market Overview of IoT for Connected Medical Devices
The IoMT market for connected medical devices (stationary, wearable, implanted) was estimated at $230.69 billion in 2024. By 2030, it is expected to reach $658.57 billion, growing at a CAGR of 18.2%. The market growth is spurred by IoT technology and advancements in medical device connectivity (e.g., 5G, Bluetooth). Among adoption drivers, there are also increasing healthcare services costs, rising prevalence of chronic diseases, the growing focus on care quality and patient safety.
How IoT for Connected Medical Devices Works
Architecture
ScienceSoft’s experts recommend the following architecture for an IoT system connecting medical devices:
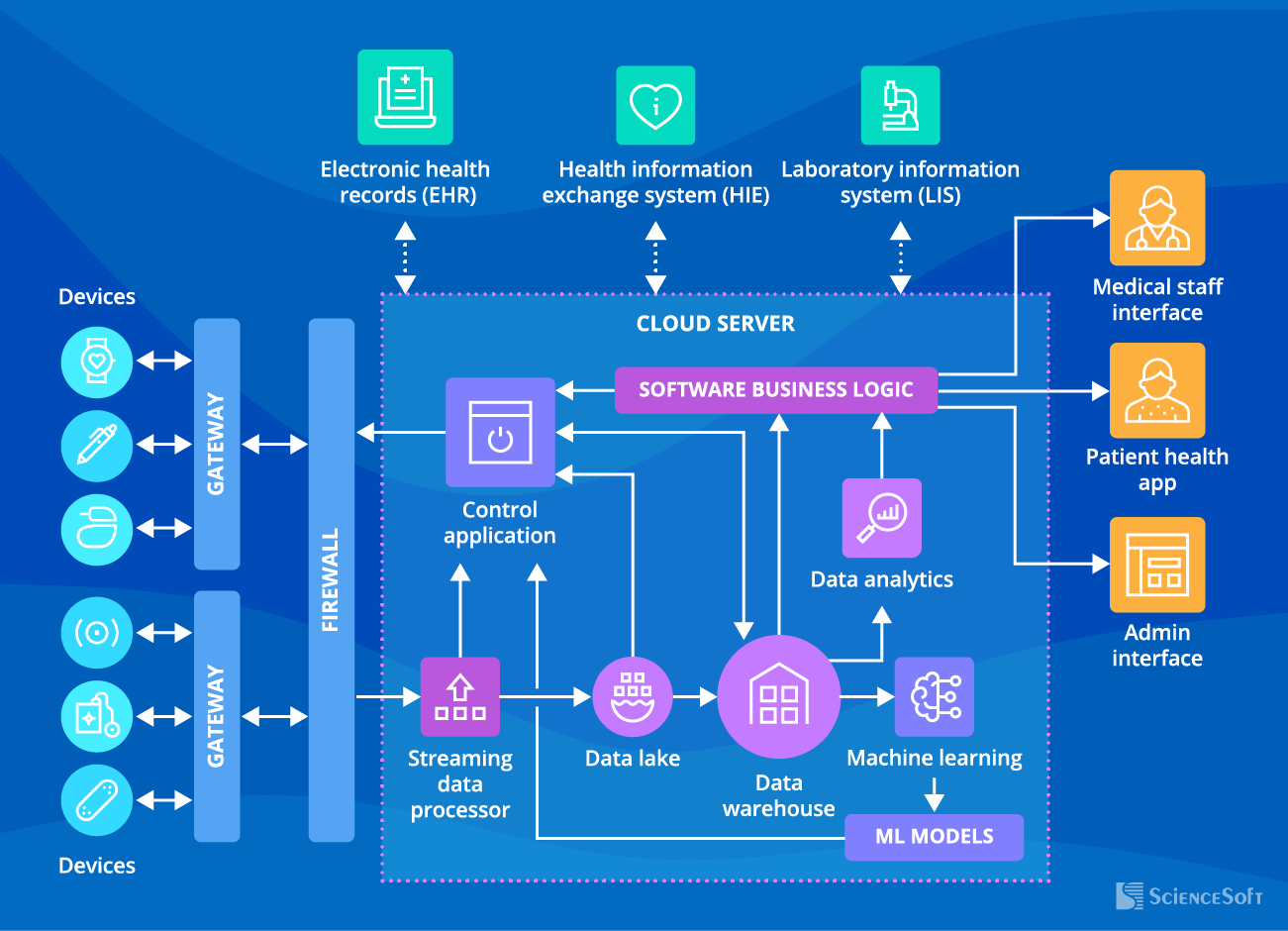
- Connected medical devices (e.g., insulin pump, ECG patch) – collect patient data to transfer it to the cloud server or deliver therapy.
- Gateways – filter, preprocess, and transmit patient data from the connected devices to the cloud; transmit control commands to connected medical devices.
- Firewall – ensures secure transmission of collected data to the cloud server.
- Streaming data processor – processes and transfers input data from the connected medical devices to the data lake and the control app.
- Data lake – stores patient data from the connected medical devices in the natural format.
- Big data warehouse – stores structured data from the connected medical devices for analysis.
- Data analytics – used to interpret data from the connected medical devices (e.g., vitals, treatment delivery, medication intake), identify trends, and deliver insights (e.g., preliminary diagnosis, recommended treatment adjustments).
- Machine learning module – used to identify patterns in patient symptoms, vitals, etc., and create ML models that power up the control application.
- Control application – triggers actions in the connected medical devices.
- Software business logic – lets patients and medical staff access data gathered by the connected medical devices, stores new configurations of medical devices and monitoring parameters, etc.
- Medical staff interface – enables medical staff to get alerts on critical changes in the patient state, configure threshold monitoring parameters for alerts, adjust a treatment plan, view patient data analytics insights, etc.
- Mobile patient health app – enables patients to review connected devices’ data (e.g., heart rate, glucose levels, device state), get alerts on suspicious health parameters, initiate consultations with medical staff, etc.
- Admin interface – enables viewing the list of current users (patients and medical staff) of the IoT system for connected medical devices, manage access to the system, etc.
Viable integrations
– for an integrated view of patients’ medical history by the care team (e.g., chronic conditions, allergies), populating medical histories with new health data, etc.
– enables a flow of patient data gathered by the connected medical devices between patients’ healthcare providers or different healthcare facilities.
– for facilitated access to the lab tests via the user apps (a patient health app, a medical staff app) of the IoMT system, etc.
Use cases
Early symptom detection
A care team can access a patient’s vitals collected over a certain period by internet connected wearable medical devices (e.g., a heart monitor, a glucose monitor). The devices may record changes in the patient’s health, which assists in patient diagnosing.
Personalized care delivery
Cloud connected medical devices and IoT technology enable remote monitoring of patients after surgery, with chronic diseases (e.g., heart failure), receiving home care, etc. ML-generated care delivery models help optimize and automate patient treatment via the implantable/wearable medical devices (e.g., insulin treatment delivery according to patients’ eating and activity habits, medication dosage adjustment).
Point-of-Care Diagnostics (POCD)
Medical staff can use connected medical devices (e.g., a blood lactate analyzer, a CRP analyzer) to conduct diagnostic procedures at a patient's home or close to it (e.g., in non-specialized local labs). Test data is automatically transferred to the IoT server for analysis and storage, and the interpreted test results are recorded in EHR/LIS.
Treatment adherence monitoring
Analysis of data from therapeutic devices (e.g., an insulin pump, an inhaler) provides insights on medication intake and compliance with the treatment plan.
Hospital disease diagnostics
Stationary connected medical devices (X-ray, CT scanners, MRI scanners, ultrasound machines) transmit medical images to the IoT server for assessment. Based on image analytics, software makes a preliminary diagnosis to enable faster and more precise medical decision-making.
Predictive maintenance of connected devices and remote troubleshooting
IoT enables remote monitoring of the technical condition of connected medical devices (e.g., CT scanners) based on incoming technical data. Using ML algorithms, the system predicts the medical device malfunctions and helps plan maintenance schedules.
Key features
In projects featuring connected medical devices, ScienceSoft ensures the following set of fundamental features and expands it further depending on the specific needs of our clients.
Real-time collection and advanced analytics of patient data
Data generated by connected medical devices (e.g., wearables, connected imaging devices) is collected and analyzed against the pre-set parameters to aid in disease diagnosing and management.
Advanced analytics capabilities help get in-depth insights into patient health, predict disease progression, etc.
IoT-based collection, storage, analytics of technical medical device data
Technical data gathered from connected medical devices (e.g., device battery state) gets analyzed against the normal parameters to identify inefficiencies and potentially dangerous malfunctions.
Alerts on abnormal parameters of connected medical devices
Medical staff (e.g., physicians, medical device technicians) and patients or their caregivers are automatically notified if vitals or device technical parameters are above or below the normal range. The alerts on potential health risks or device failure are sent to a medical staff app or a patient app.
Remote configuration of medical devices
Using collected data and ML-identified patterns in patients’ health data or device functioning, the care team can configure patient monitoring parameters, device settings, perform device check and maintenance remotely, etc.
Technology Elements of an IoT System for Cloud Connected Medical Devices
Since 2005 in healthcare IT, ScienceSoft offers opting for the following reliable techs to develop an IoT system for connected medical devices.
Device connectivity
Cloud services
Real-time data streaming

Data lakes
Challenges of IoT for Connected Medical Devices and How to Tackle Them
Challenge #1
To create a full-fledged IoT system for medical devices, a software product company or a medical device manufacturer requires the considerable expenses on a highly qualified tech team.
Solution
Challenge #2:
Cloud connected medical devices evoke concerns about the security and privacy of transmitted data.
Solution
Implementation Costs
General cost drivers
- The number and complexity of IoT software features for connected medical devices.
Show more
Additional cost drivers
- Hardware costs (costs of medical devices, sensors for device connectivity, etc.).
Operational cost drivers
- Usage of cloud services (e.g., for cloud data storage, data analytics).

According to ScienceSoft’s experience, the cost of an IoT solution for connected medical devices starts from $200,000 to $250,000+.
Need a price tag on your project?
Estimate the Cost of Your Medical Device Software
Please answer a few questions to help our healthcare IT consultants accurately assess your needs and calculate a personalized quote quicker.
Thank you for your request!
We will analyze your case and get back to you within a business day to share a ballpark estimate.
In the meantime, would you like to learn more about ScienceSoft?
- Project success no matter what: learn how we make good on our mission.
- Since 2005 in healthcare IT services: check what we do.
- 4,200+ successful projects: explore our portfolio.
- 1,400+ incredible clients: read what they say.

IoT for Connected Medical Devices: ScienceSoft’s Consulting and Development Services
ScienceSoft brings 14 years of experience in IoT and 20 years in healthcare to provide you with reliable IoT software connecting a range of medical devices. You set goals, we drive the project to fulfill them in spite of time and budget constraints, as well as changing requirements.
IoT for connected medical devices: consulting
ScienceSoft’s team will:
- Plan the functionality of IoT software connecting medical devices based on the analysis of your business needs, requirements, etc.
- Design a high-level architecture of the IoT system.
- Detail necessary integrations with medical IT systems and connected medical devices.
- Define applicable interoperability (e.g., HL7, FHIR, CCDA, USCDI) and terminology (e.g., SNOMED CT, LOINC) standards.
- Create a business case, estimate the cost, ROI of the IoMT system.
- Plan IoMT software delivery schedule and timelines.
- Offer a step-by-step compliance plan (e.g., for HIPAA, Cures Act, GDPR, FDA, or MDR/IVDR requirements).
Development of IoT systems for connected medical devices
ScienceSoft’s team will:
- Conceptualize IoT software for connected medical devices based on your high-level or detailed requirements.
- Draw up a comprehensive IoT software feature list.
- Create a flexible and scalable IoMT software architecture and ensure integration with medical devices.
- Develop and test the IoMT MVP with priority features.
- Roll out other software functionality upon the agreed schedule (with 2-4 week iterations).
- Ensure compliance of the IoT system for connected medical devices with required regulations (HIPAA, HITECH, etc.).
- Provide IoMT software maintenance and evolution services (if required).
About ScienceSoft
Headquartered in McKinney, TX, ScienceSoft provides professional healthcare software design and development services since 2005. We hold ISO 13485 certification and create medical IoT software for connected devices according to the requirements of the FDA and the Council of the European Union.