AI-Powered Medical Devices
Architecture, Functionality, Use Cases
ScienceSoft leverages 36 years of experience in AI software development and 20 years in healthcare IT to create AI-enabled medical software that our clients can rely on.
AI-Powered Medical Devices: The Fundamentals
AI-based medical devices comprise two main categories: medical devices connected to AI-enabled cloud software and AI-supported applications that function as medical devices. In both cases, artificial intelligence can significantly improve healthcare outcomes by assisting doctors with diagnostics, treatment planning and management, complications prediction, drug prescription, dosage calculation, and more.
Market Overview
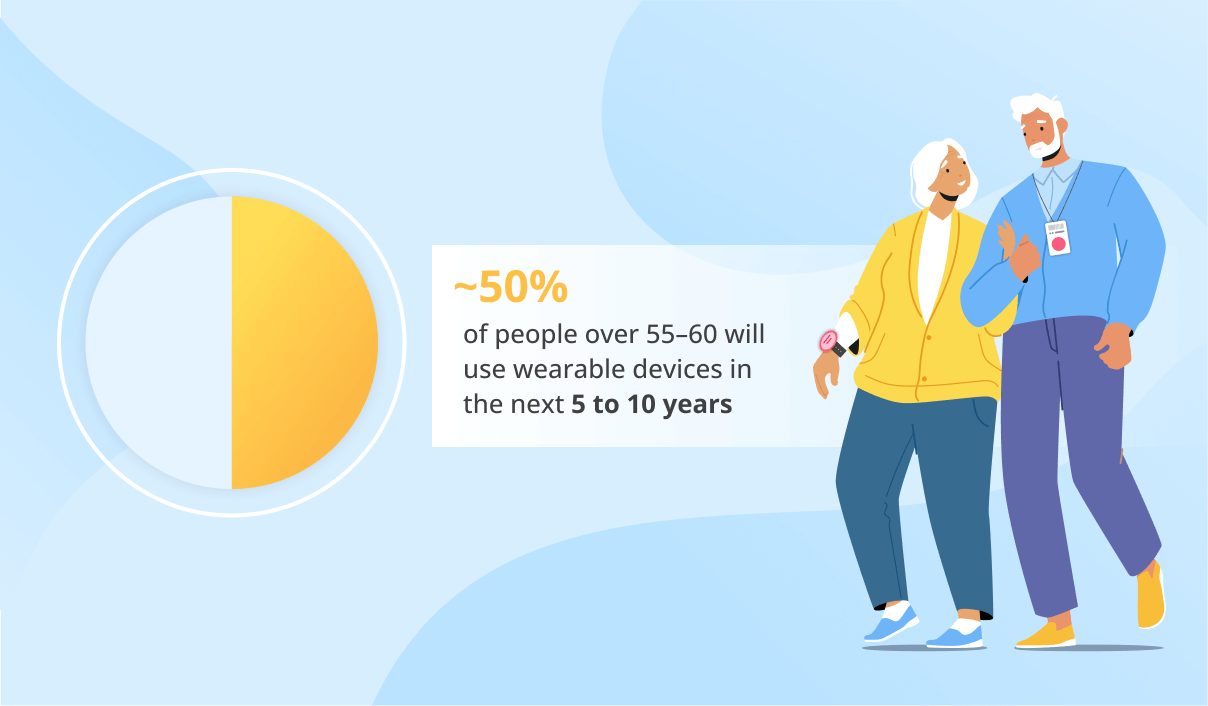
By 2033, the AI/ML-powered medical devices market is expected to reach $255.76 billion.
The vast majority of devices authorized as of 2024 are in radiology, followed by cardiovascular, neurology, hematology, gastroenterology/urology, ophthalmology, and anesthesiology.
How AI-Powered Medical Devices Work
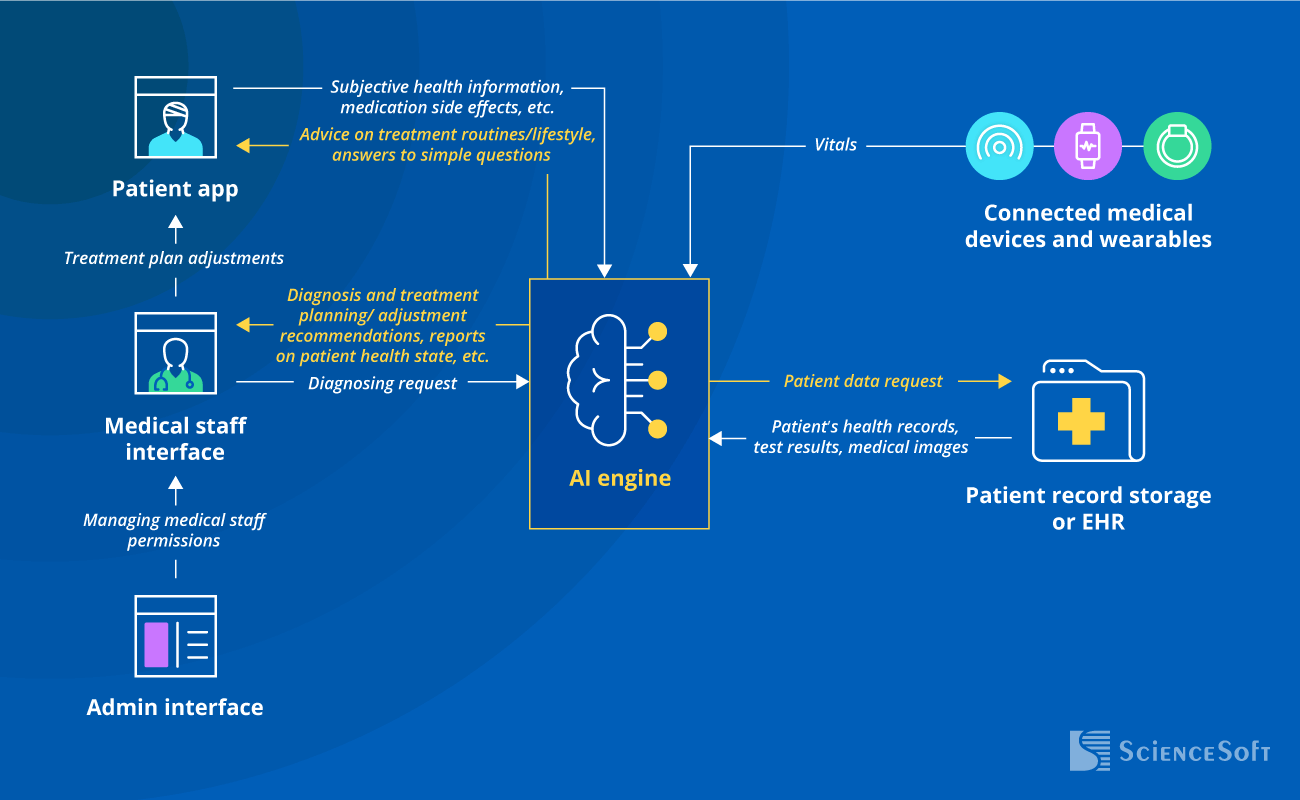
Architecture
This is a sample architecture of an AI-supported SaMD solution recommended by ScienceSoft’s consultants.
Use cases
When connected to the ERP system, AI-supported SaMD with pattern recognition and computer vision capabilities can analyze large amounts of clinical data from various sources (lab tests, medical images, etc.). This helps identify unique dependencies or abnormalities that could be overlooked by a human. The system then generates a list of possible diagnoses.
AI-enabled SaMD integrated with the ERP system evaluate unique patient characteristics such as medical history, genetics, and current physiological data to generate a tailored list of treatment recommendations or potential medications that could be reviewed and modified by the doctor. In addition, the integration with the patient app and smart patient wearables enables disease progression analysis and proactive treatment adjustment.
Remote care delivery
The integration of AI with smart wearable or implanted medical devices (e.g., glucose monitors or ECG patches) can enhance the accessibility and continuity of care for those unable or unwilling to visit the hospital in person. AI-supported solutions facilitating care delivery also include chatbots to interview patients on their conditions and symptoms or provide information for self-help, e.g., recommend first-aid measures.
Medication efficiency monitoring
AI-powered cloud software can use data captured from smart medical devices like pill bottles or insulin pens to assess medication efficiency and recommend alternatives or dosage adjustments.
Potential features
Below are some of the features that could be implemented, depending on the software purpose:
Processing data from multiple sources
Heterogeneous clinical data from EHR, laboratory results, patient-generated data, etc., is automatically collected via FHIR-based APIs into a unified dataset stored in a HIPAA-compliant cloud and available for analysis.
Predictive clinical data analysis
Analysis of vitals, lab tests, medical images, etc. to identify patterns indicative of certain diseases and predict disease progression and risks.
Transforming spoken words into text data to enable streamlined recording processes, real-time analysis of patient-generated data, and easier accessibility for users with disabilities.
Processing medical images of any type (CT, MRI, ultrasound, etc.) to enable the detection of abnormal areas that may go unnoticed by a radiologist or a physician.
Interviewing patients on their symptoms, providing information or tools for self-help, e.g., tips for anxiety relief.
Decision support
Smart treatment suggestions for doctors based on individual patient characteristics.
Data protection measures
Access control, unique user identification, user authentication, data encryption to ensure compliance with HIPAA, GDPR, and other applicable regulations.
Chest X-Ray AI Feature for SaMD
This short demo shows a diagnostic AI feature inside a SaMD product for chest X-rays. A clinician pulls the study from PACS, reviews probability-backed findings with heat maps, and exports a report draft in one click. The solution is built for 90% or higher diagnostic accuracy and roughly 30% shorter reads.
Technologies ScienceSoft Uses to Develop AI-Powered Medical Software or SaMD
Machine learning platforms and services
AWS





Azure
Google Cloud Platform
Machine learning frameworks and libraries
Frameworks
Libraries
Challenges and Concerns
Does my app need FDA approval?
According to FDA, software that is “unrelated to the diagnosis, cure, mitigation, prevention, or treatment of a disease or condition” is not a medical device. If a product is only dedicated to maintaining or encouraging a healthy lifestyle, it’s considered a general wellness product and, therefore, does not require submission to FDA.
Ensuring the accuracy of ML models.
As a first step, data scientists should ensure that data sets intended for ML model training are of high quality. This can be achieved by examining and cleaning the data to avoid erroneous, missing, or inconsistent values. During the ML model training, continuous testing for the credibility of the results is essential. At ScienceSoft, data scientists use, for example, dropout techniques and optimization algorithms to minimize the model’s output error.
Building a custom model from scratch can be quite costly.
Custom AI/ML models might be feasible in case of, for example, medical diagnosing where precision and security are crucial. For more common tasks such as speech recognition, ScienceSoft’s data scientists suggest using pre-trained models. This will be a more cost-effective and faster to implement option than custom ML algorithms. Another solution would be tailoring and re-training an existing model.
How Much Does It Cost to Implement AI-Enabled SaMD or Software for Medical Devices?
When determining the cost of AI-supported medical software development, ScienceSoft considers the following factors:
- Functional scope and complexity defined by the software purpose (diagnostics, chronic disease management, etc.).
- Complexity of AI functionality (e.g., a chatbot that provides information upon request vs. image analysis system leveraging deep learning algorithms).
- Performance, scalability, security, and compliance requirements for the software.
- Number and complexity of integrations with other systems (EHR, doctor/patient apps, etc.).
- Deliverable type (PoC, MVP, a fully-featured solution).

From ScienceSoft’s experience, the cost of AI-powered cloud software for medical devices or AI-enabled SaMD may range from $200,000 to $650,000+. The former could be relevant for an individual AI-supported module (e.g., for drug dosage calculation), while the latter applies for end-to-end systems with several role-specific app integrations and complex algorithms dealing with diverse medical data.
Need a tailored cost estimation for your medical device software?