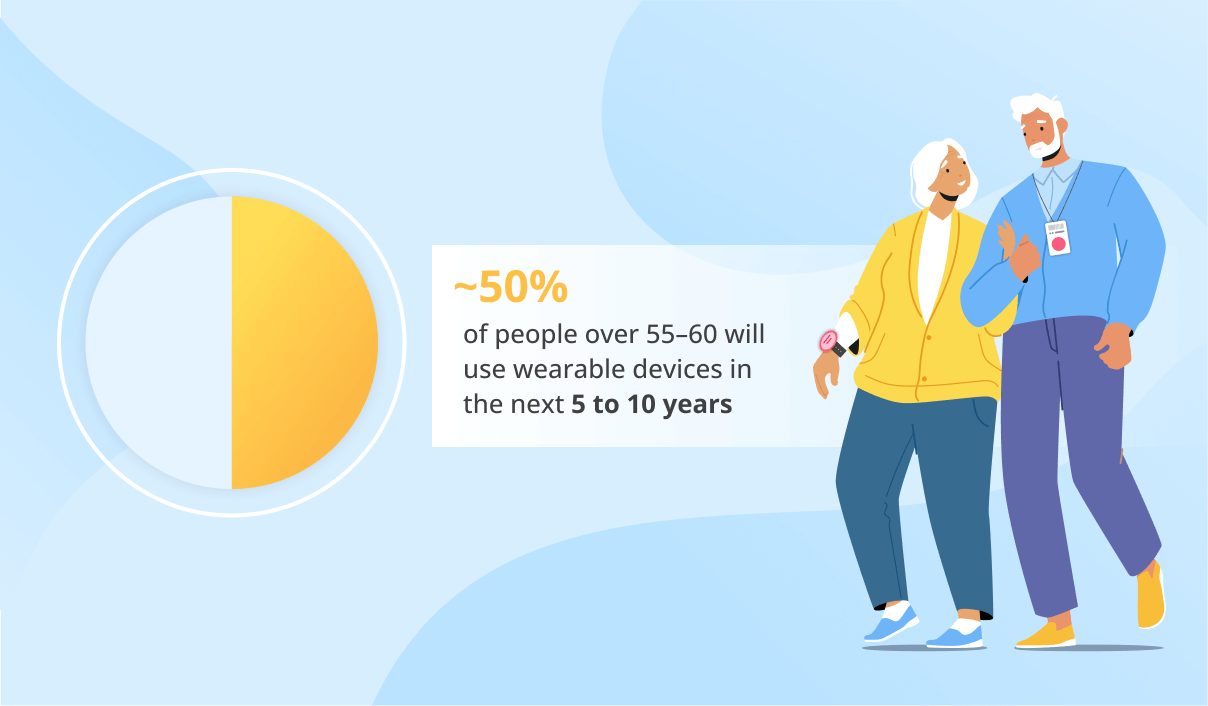
Medical Device Software Development Services
Medical device software development services by ScienceSoft are backed by ISO 13485 certification and 20 years of experience in healthcare. We rely on proven software architecture and project management practices to design and build secure FDA-cleared SaMD and software for medical devices.
What Makes ScienceSoft a Reliable Tech Partner
- Medical device software development company with since 1989 in IT and since 2005 in the healthcare domain.
- Tailored healthcare software addressing specialized workflows, patient-facing applications, and facility-specific requirements.
- ISO 13485, ISO 9001, and ISO 27001 certifications.
- Knowledge of healthcare standards (HL7, ICD-10, CPT, XDS/XDS-I, etc.).
- Experience in FDA registration and CE marking, HIPAA and HITECH-compliant software development for medical devices.
- Field-tested healthcare software quality management and security management approach.
- Doctor of Medicine healthcare IT consultant on board.
- Microsoft partner since 2008.
- International software development company with HQ in Texas, US, and offices in Europe and the Gulf Cooperation Council.
Our awards, recognitions, and certifications
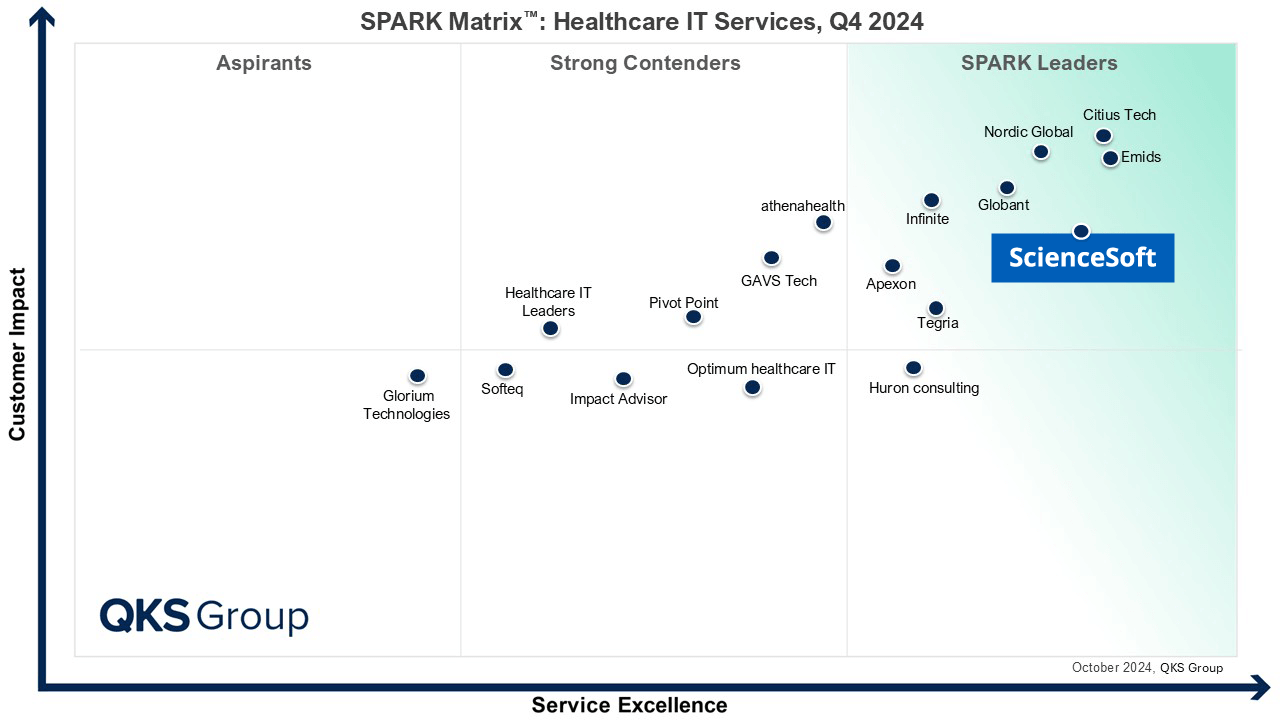
Featured among Healthcare IT Services Leaders in the 2022 and 2024 SPARK Matrix
Recognized for Healthcare Technology Leadership by Frost & Sullivan in 2023 and 2025
Named among America’s Fastest-Growing Companies by Financial Times, 4 years in a row

Top Healthcare IT Developer and Advisor by Black Book™ survey 2023
Four-time finalist across HTN Awards programs

Named to The Healthcare Technology Report’s Top 25 Healthcare Software Companies of 2025

HIMSS Gold member advancing digital healthcare
ISO 13485-certified quality management system
ISO 27001-certified security management system
Medical Device Software ScienceSoft Delivers
Software for medical devices
Target medical devices (including wearable and implanted devices):
- Class II devices – medium-risk medical devices (CT scanners, wearable glucose monitors, blood pressure monitors, wearable ECG sensors, etc.).
- Class III devices – high-risk medical devices (pacemakers, cardioverter-defibrillators, deep-brain stimulators, etc.).
Sample use cases:
- Remote monitoring of patients with chronic diseases (e.g., COPD, diabetes, cardiovascular, neurological conditions).
- Remote patient care delivery via smart therapeutic devices (e.g., insulin pens, smart inhalers).
- Early disease diagnostics using medical devices or sensors, data analytics, AI/ML.
- Medication intake monitoring (using smart pill bottles, etc.).
- Hospital conditions monitoring and adjusting.
Software as a Medical Device (SaMD)
Target devices:
- Smartphones, tablets.
- PCs, laptops.
- Smartwatches and wearable fitness trackers.
- Smart TV.
Sample use cases:
- AI-based disease treatment and patient care planning.
- ML-based drug prescription recommendation system for doctors.
- Drug dosage calculation.
- Medical image viewing (DICOM viewer).
- Disease diagnosing via image recognition (e.g., for stroke type identification, brain tumor localization).
- Direct disease management (e.g., sleep apnea episode identification, sound therapy for tinnitus patients, reminiscence therapy for patients with Alzheimer’s disease).
Most Requested Modules for Medical Device Software
Mobile app
On-the-go access to medical device software or SaMD data helps ease the work of healthcare professionals or the disease management for the patients.
Advanced analytics
Using AI and ML, the medical device software uses data from connected devices to identify patient trends and predict the course of the disease, detect hazardous symptoms at an early stage, assess medication efficiency, etc.
Cloud platform
Cloud-connected medical devices (e.g., glucose monitors, pacemakers, smart inhalers) transmit patient vitals, diagnostics, and treatment data to the cloud platform for analytics. Cloud platform enables management of the connected devices (e.g., device failure identification, parameters configuration).
Complex medical equipment and devices are tracked to identify their locations, ensure proper device disinfection and sanitation, decrease device search time, and provide remote maintenance.
Real-time health monitoring
To automate the hospital workflows and motivate patients to manage their health, the medical device software collects, stores, and analyzes patient health information (e.g., blood glucose level, sleep data).
Should you consider cloud-based integration for your medical device software?
Along with effectively enabling communication of medical devices with healthcare software, implementation of data analytics, and predictive device maintenance, cloud integration is highly secure. Cloud providers (AWS, Microsoft Azure) comply with HIPAA requirements and guarantee the security of stored PHI.
To further enhance safety, ScienceSoft ensures data anonymization and encryption as well as conducts regular vulnerability assessment and penetration testing.
Our Medical Device Software Development Processes
1
Needs elicitation, requirements gathering, and specification approval
We discuss the relevant needs and the idea of medical device software with key stakeholders on your side to coin the software concept, identify and prioritize software requirements. Then, we create a detailed specification of medical device software, analyze software risks and create a risk management plan.
2
Medical device software engineering
We create a reliable and scalable architecture (e.g., N-tier, SOA) for your medical device software that allows adding new modules/device types with little to no rework. Our architects ensure system configurability, clear module interfaces, and good encapsulation of every module.
3
UX/UI design of medical device software
We conduct UX research, design user scenarios, and map comprehensive user journeys. Sleek medical device software UI enables fast and easy software adoption by all age groups regardless of their tech-savviness.
4
Medical device software development
When developing medical device software, ScienceSoft’s team guarantees:
- Cross-platform development to cater to multiple operating systems.
- Seamless integration with healthcare software (EHR, ADT) via HL7 v.3 or FHIR.
- Smart methodology choice: Waterfall – full-fledged version delivered in one iteration; Iterative (Agile, Scrum) – MVP delivery with updates every 2-4 weeks.
5
Quality assurance
ScienceSoft applies OWASP’s S-SDLC (Secure Software Development Life Cycle) practices that involve comprehensive multi-level quality assurance. Striving for 100% code coverage, we conduct continuous testing, software validation and verification, and regular code reviews during the development.
6
Premarket submission
To meet the requirements of the FDA and the Council of the European Union and ensure software safety, we provide:
- Development services according to ISO 13485, IEC 62304, and IEC 82304-1.
- Detailed documentation (e.g., Design History File) for FDA 510 (k) premarket notification, CE marking, HIPAA compliance audits, etc.
- HIPAA/GDPR risk assessment.
- Guidance through FDA/CE/MDR/IVDR submission process.
7
Integration with smart devices (wearable and non-wearable, medical or general-purpose)
We enable smooth interaction of the software with devices and guarantee:
- Stable communication with the IoMT system (e.g., for remote patient monitoring).
- Comprehensive analytics of patient-generated health data collected by devices.
8
Medical device software support and evolution
If required, we provide medical device software support, manage the performance and security of your software, perform routine software administration tasks, and help your medical device software evolve further.
Advanced Technologies We Are Experienced In
Click on the cards below to learn more about ScienceSoft’s skills in trending technologies.
Software That Will Benefit from Integration with Medical Devices
RPM apps and apps for medical staff
Care teams have real-time access to automatically gathered vitals of hospital and remote patients and get alerts in case of critical changes.
Physicians can accurately guide remote care based on precise health data collected by devices and sensors.
To help patients conveniently monitor their chronic conditions (e.g., daily insulin intake or blood glucose level for diabetic patients).
By collecting data from stationary and portable medical devices, EHRs present a holistic medical history for patient diagnostics and treatment.
Technologies ScienceSoft Uses
Device connectivity
Cloud services
Real-time data streaming

Data lakes
Costs of Medical Device Software Development
Major factors that affect the development budget and timelines are:
- The medical device software solution’s functional scope.
- The number and complexity of integrations with healthcare devices.
- Performance, scalability, security, and compliance requirements for the software.
- The required development scope (PoC, MVP, a fully-featured solution).
- Sourcing model and team composition.
- (for outsourced development) A vendor’s service pricing model.
Based on ScienceSoft’s experience in healthcare IT, building custom medical device software may cost around $200,000–$800,000+.
Estimate the Cost of Your Medical Device Software
Please answer a few questions to help our healthcare IT consultants accurately assess your needs and calculate a personalized quote quicker.
Thank you for your request!
We will analyze your case and get back to you within a business day to share a ballpark estimate.
In the meantime, would you like to learn more about ScienceSoft?
- Project success no matter what: learn how we make good on our mission.
- Since 2005 in healthcare IT services: check what we do.
- 4,200+ successful projects: explore our portfolio.
- 1,400+ incredible clients: read what they say.

Pricing options ScienceSoft offers
Fixed price (FP)
We pre-agree on the fixed quote and the payments are bound to the project’s milestones. This model is best for medical device software projects with no expected changes.
Time and Materials (T&M)
Monthly, we issue a report on the completed tasks and charge you in accordance with the progress made. This option is optimal if the project scope is prone to requirement changes.
Time and Materials with a cap (T&M NTE)
As in T&M, we charge you an hourly rate of outsourced specialists for the time spent on medical device software development every month. Yet, there is a fixed maximum total charge.
Challenges We Solve
|
|
Intricate compliance procedures and certification process We know all the ins and outs of compliance procedures and will carefully guide you. Being ISO 13485-certified, ScienceSoft will prepare a full package of information for software clearance by the notified bodies according to FDA and MDR requirements. Also, we are always open for an audit of our processes, so you can be sure of the quality of our services. |
|
|
Slow market entry With us, you won't lose the momentum: we are quick to react to your needs and will deliver an MVP in six months or less. We know that time-to-market is critical and we follow the pre-agreed timelines without compromising the software quality. |
|
|
Device integration issues ScienceSoft's engineers will streamline the medical device or SaMD integration without any additional efforts on your or your end customers' side. Besides, integrating new device models and types will require little to no rework thanks to the flexible architectures we build. |
|
|
Vendor lock-in You are free to choose if you want to partner with us for further software support and maintenance or to shift to another vendor. You'll be fine either way because we provide well-documented project code and tests and conduct knowledge transfer, if necessary. |
Questions That May Still Bother You
What's the difference between a medical device and SaMD?
A medical device is an apparatus, machine, implant or any other device performing diagnostic or treatment procedures. SaMD is a purely digital solution that operates on a general-purpose device (like a smartphone or a PC) or is device-independent (e.g., an AI assistant that spots potential complications). SaMD can use device-generated data (e.g., to predict blood sugar levels based on glucometer readings) or rely on device functionality to diagnose or treat the disease (e.g., use smartphone's speakers to deliver tinnitus therapy).
Does SaMD require approval from the FDA or similar agencies?
Yes, it does. To make sure SaMD is as safe and efficient as any medical device, the FDA set up an approval process. It varies based on the SaMD safety class, with the most common procedure being FDA 510(k) submission. In the EU, according to MDR, SaMD must be approved by respective local agencies in each member state. If you have doubts regarding the applicable regulations, just drop us a line!
Are systems like EHR considered SaMD?
No, systems that collect and store patient information are not considered SaMD unless they have advanced functional modules used for automated diagnosing or treatment planning (e.g., AI medication dosage calculation or medical image analysis). Such modules of advanced EHR are subject to a standard submission to the FDA or local authorities.
Benefits of Cooperation with ScienceSoft
|
Cooperation without large upfront investments due to iterative development. |
Delivery on time and within budget thanks to DevOps and balanced manual & automated testing. |
|
UX design that passed usability testing, to ensure fast adoption by all age groups. |
Prevention of software bugs and malfunctions to guarantee flawless device performance. |
|
High-quality software architecture and code, allowing for cheap and easy maintenance. |
Assistance with preparing all the necessary documents for FDA registration submission. |
ScienceSoft’s Approach to Service Delivery
Transparency and KPIs
We keep all our medical device software development activities open to audits from your side. We may discuss and set KPIs (e.g. test coverage, customer satisfaction, etc.) that we share with you in regular reports to ensure the project is on track.
Rapid project delivery
We are ready to kick off a project within a week. To develop a medical device software MVP with priority features, we need 3-6 months. Then, we roll out other features according to the pre-agreed schedule with major releases every 2-4 weeks.
Post-delivery support
If needed, we help with applying for FDA/CE registration of medical device software or SaMD by preparing all necessary developer documentation for you. We also provide continuous software support and offer software evolution.