Medical Device Software Testing
Be Confident in Your Medical Device Software
Since 2005 in healthcare IT and since 1989 in testing, ScienceSoft helps medical device manufacturers and software providers ensure the quality of medical device software and its compliance with regulatory standards.
Medical device software testing is a way to guarantee the solution’s quality by verifying its requirements and architecture and checking if it meets user needs.
ScienceSoft’s Testing Center of Excellence has accumulated working best practices for testing medical device software. Our team will eagerly implement them to help you smoothly introduce your software to the market or confirm its compliance.
Scope of Our Medical Device Software Testing Services
Companies we serve
- Medical device manufacturers.
- Medical device software providers.
- Healthcare providers.
- Pharmaceutical companies.
Medical devices we test
- Class II – medium-risk medical devices (CT scanners, wearable glucose monitors, blood pressure monitors, wearable ECG sensors, etc.).
- Class III – high-risk medical devices (pacemakers, cardioverter-defibrillators, deep-brain stimulators, etc.).
Testing types we excel at
- Functional, including integration and regression testing.
- Usability testing.
- Compatibility testing.
- Performance testing.
- Security testing.
Our testing approaches
We Know the Importance of Thorough Documentation
ScienceSoft understands that you need complete and comprehensive test documentation and provides you with the following deliverables:
|
|
Who We Are in Numbers
Why Companies Trust Us
- ISO 13485:2016 certified to guarantee testing compliance with the requirements of the FDA and the Council of the European Union.
- ISO 27001 certified to guarantee the security of sensitive information and test data.
- Team with hands-on experience in HIPAA, HITECH and FDA requirements and healthcare data exchange standards (e.g., HL7, ICD-10, CPT, XDS/XDS-I).
- Experience in FDA/CE registration.
- ISTQB-certified test engineers and Doctor of Medicine consultants on board.
Our awards, recognitions, and certifications
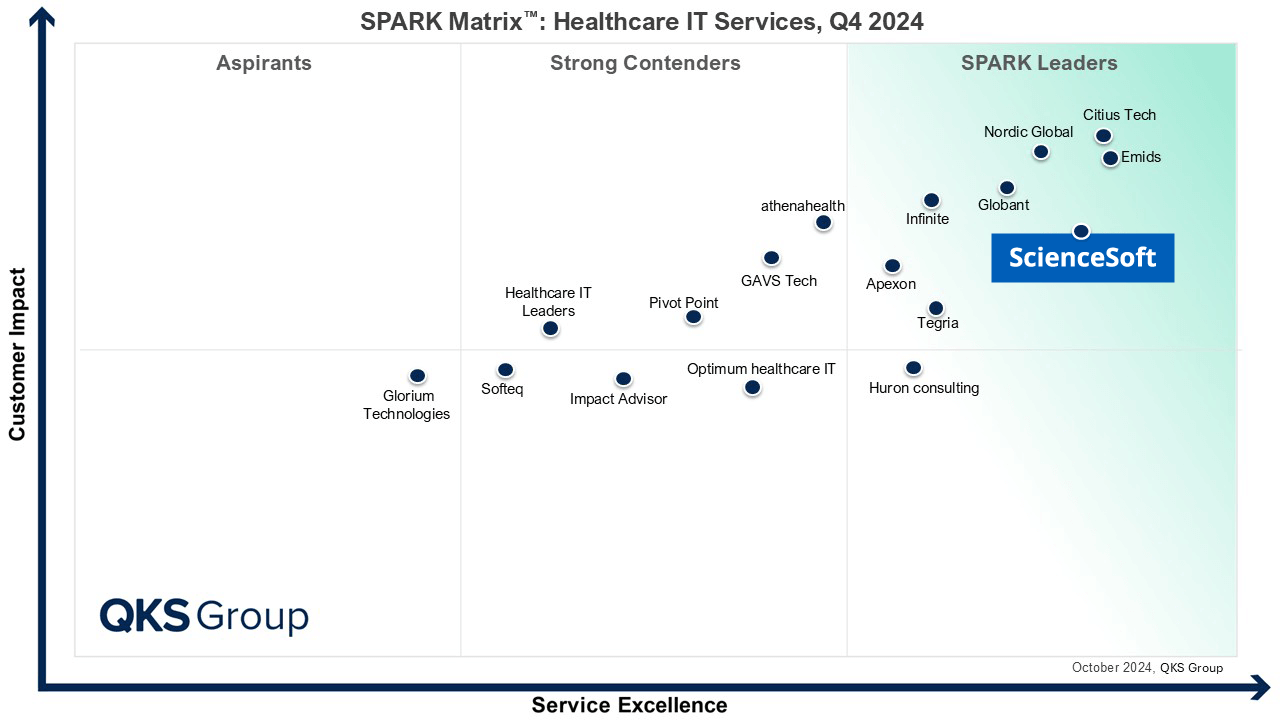
Featured among Healthcare IT Services Leaders in the 2022 and 2024 SPARK Matrix
Recognized for Healthcare Technology Leadership by Frost & Sullivan in 2023 and 2025
Named among America’s Fastest-Growing Companies by Financial Times, 4 years in a row

Top Healthcare IT Developer and Advisor by Black Book™ survey 2023
Four-time finalist across HTN Awards programs

Named to The Healthcare Technology Report’s Top 25 Healthcare Software Companies of 2025

HIMSS Gold member advancing digital healthcare
ISO 13485-certified quality management system
ISO 27001-certified security management system
Benefits of Testing with ScienceSoft
-20-40% testing costs
due to:
- KPIs-based testing, tailored to software specifics.
- Reusable test cases.
- Prioritization of testing activities based on the software intended use and risks.
Zero penalties
due to:
- 100% regulatory compliance of medical device software.
- Lack of software-driven malfunctions due to comprehensive testing.
Decreased rate of medical device recalls
due to:
- Result-oriented and thorough testing of your medical device software.
- Complete and comprehensive test documentation.
Having Doubts About Third-Party Testing? Let Us Change Your Mind!
During medical device software testing, there is a risk of disclosing patients’ health information and breaching HIPAA regulations. How can a third-party vendor handle security issues?
During testing projects, we at ScienceSoft apply VPNs, SSL certificates, encryption protocols, etc. The safety of our internal infrastructure is confirmed by ISO 27001 certification.
Will an outsourced testing team be able to swiftly dive into the specifics of our medical device software?
ScienceSoft has multiple successful projects in medical software testing under its belt and guarantees a quick transition. We effectively collaborate with other teams involved in your project so that no business or technical specifics get missed.
Apart from that, ScienceSoft’s testing engineers have working experience with major healthcare regulations (HIPAA, HITECH, ONC, MACRA, MIPS, CEHRT, SAFER) and healthcare data exchange standards (e.g., HL7, ICD-10, CPT, XDS/XDS-I).
Will we be able to track the testing process of our medical device software?
ScienceSoft ensures full transparency over the testing activities, offering detailed reporting and providing test documentation required by ISO 13485 and ISO/IEC/IEEE 29119-3:2021.
We Can Cover Any Testing Need
ScienceSoft’s healthcare testing professionals will:
- Verify the quality of medical device software requirements.
- Test medical device software at the API, integration, and system levels.
- Verifying that software architecture is secure.
- Perform software code review and code analysis.
- Run user acceptance testing.
Compliance testing
ScienceSoft’s compliance experts will help ensure medical device software complies with required standards and regulations (e.g., FDA, HIPAA).
Tools We Use to Test Software for Medical Devices
Automated mobile testing tools
Performance testing tools
API testing
Security testing tools
Automated UI testing tools
CI/CD tools
Test management and defect tracking software
