Cardiology Device Software Development Services
Companion Apps and SaMD
With 20 years of experience in medical software engineering, ScienceSoft helps OEMs launch reliable, compliant companion software and SaMD alongside their cardiology devices. Our architects design solutions that integrate with real-world hospital systems and scale with product demand, even in the face of evolving regulations or tight launch timelines.
Cardiology device software development services include the design, development, modernization, support, and evolution of software solutions that accompany medical devices used in cardiology. These services cover the development of clinical, operational, and research-focused software such as remote cardiac monitoring platforms, diagnostic applications (incl. SaMD), device fleet management systems, clinical trial portals, and direct-to-consumer mobile apps.
ScienceSoft as a Trusted Partner for Cardiology Medical Device Software Development
- Since 2005 in medical software engineering with 150+ successful projects.
- Since 2011 in IoT and since 2012 in cloud technologies.
- Proficiency in achieving and maintaining compliance with the requirements of HIPAA/GDPR, IEC 62304, ISO 13485, 21 CFR Part 820, 21 CFR Part 11, and more.
- An official partner of Microsoft and AWS.
- 9 principal architects with 15–25+ years of experience each to balance the security, cost-efficiency, and longevity of IoMT architectures.
Our awards, recognitions, and certifications
Featured among Healthcare IT Services Leaders in the 2022 and 2024 SPARK Matrix
Recognized for Healthcare Technology Leadership by Frost & Sullivan in 2023 and 2025
Named among America’s Fastest-Growing Companies by Financial Times, 4 years in a row

Top Healthcare IT Developer and Advisor by Black Book™ survey 2023
Four-time finalist across HTN Awards programs

Named to The Healthcare Technology Report’s Top 25 Healthcare Software Companies of 2025

HIMSS Gold member advancing digital healthcare
ISO 13485-certified quality management system
ISO 27001-certified security management system
Cardiology Device-Connected Software We Develop
These systems are designed to monitor and manage wearable ECG devices prescribed to patients by their healthcare providers. They collect cardiac signals and securely transmit them back to clinicians. Heart monitoring device software then processes and analyzes incoming data to detect clinically relevant events like arrhythmias or irregular variability, and notifies care teams when follow-up is needed.
Cardiology diagnostic software
Clinicians use device-connected diagnostic platforms to analyze patient cardiac signals — for example, ECG recordings collected during in-clinic procedures or downloaded from portable monitors or event recorders. These solutions support signal visualization, automatic detection of rhythm abnormalities, annotation, and report generation. Some OEMs build workstation-based tools that allow technicians to upload, view, and process device data offline.
Cardiology data review and reporting software
Clinical and research teams use these web and desktop tools to upload device recordings, view multi-lead waveforms with filters and zoom, review algorithm-marked events, annotate segments, and generate structured reports. Specialized dashboards can display clinically relevant trends (e.g., event burden over time, heart rate variability patterns, signal quality) and enable secure export or transfer of reports and data to downstream systems.
OEMs often offer companion software platforms that enable providers’ technical teams to track the location and condition of devices deployed across their facilities. These platforms support device registration, location tracking (e.g., via RFID or BLE), real-time status monitoring, and maintenance logging. They often integrate with service ticketing or inventory systems and may include dashboards for viewing usage patterns, service history, or error events across departments or sites.
Direct-to-consumer apps
Direct-to-consumer companion apps are designed for individuals using cardiology devices outside of a clinical setting. These apps display personal metrics such as resting heart rate, irregular rhythm flags, and heart rate variability trends based on periodic recordings. Users can view data over time, receive reminders, and export records to share with their healthcare provider.
Clinical study data portals
Specialized portals can support clinical studies that use cardiology devices as a data source. Using these portals as a device-connected layer alongside eClinical tools (e.g., EDC/CTMS/eTMF), sites can securely upload and QC device data, track adherence, and manage queries. Investigators, monitors, and sponsor teams can access role-based dashboards and ready device data exports.
High-Level Architecture of a Remote Cardiac Monitoring Platform
Below is a high-level architecture diagram for a remote cardiac monitoring solution deployed entirely within a hospital’s infrastructure. This setup suits providers with strict privacy or data residency policies that prevent protected health information (PHI) from being processed outside their own IT environment. However, the same functional architecture can be implemented in cloud or private cloud modes. In each of our projects, ScienceSoft’s architects define the deployment model early to align integrations, PHI handling, and validation scope.
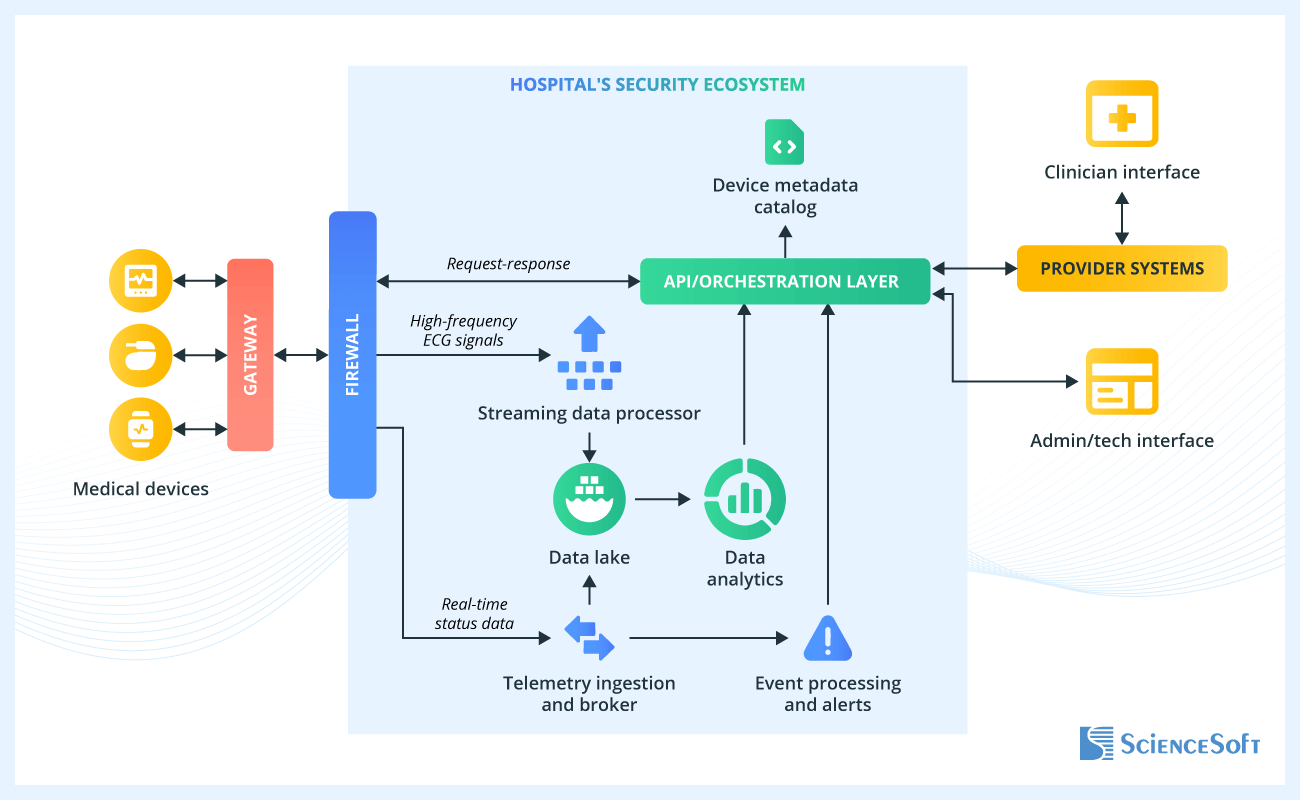
In the reference architecture, medical devices (e.g., wearable ECG sensors) are used by patients outside the hospital to continuously collect cardiac signals. These devices connect to a dedicated gateway, which securely transmits the data to the hospital’s infrastructure, separated by a firewall.
Inside the protected environment, high-frequency signals pass through the streaming processor, which segments and normalizes incoming data and detects clinically significant anomalies (such as atrial fibrillation or abnormal rate variability). Both signal fragments and initial event markers are stored in the data lake, where they remain available for further analytics. In parallel, real-time status data such as connectivity and signal quality is routed through a telemetry broker and also stored in the data lake for long-term tracking.
The data lake feeds data into the data analytics layer, which runs device‑specific or clinical algorithms, generates insights, and supports dashboards for different user groups. Events, whether from immediate stream-level detection or retrospective analytics, are passed to the event processing and alerting module, which routes actionable information to the appropriate personnel.
The API and orchestration layer coordinates all platform modules, managing data and event flows, integrating with other hospital systems (e.g., EHR or cardiovascular information system), and enforcing access policies. It also serves as the connection point for the device metadata catalog, which maintains operational records for monitored devices. The orchestration layer also handles secure device onboarding (provisioning, pairing, and device identity), configuration and threshold updates, certificate/key rotation, and device lifecycle events such as replacement and decommissioning. For connected devices that support it, the same control plane can manage firmware update distribution and reporting of update status.
Medical and non-medical users can access the system via the clinician interface or the admin or technician interface, respectively.
The entire solution can be delivered as a containerized or virtualized package, allowing vendors to deploy the same setup across multiple customers and ensuring that all protected health information (PHI) is stored and processed within the provider-controlled environment.
Services We Offer
Cardiology software product consulting
We assess your device’s use cases, target environments, and compliance landscape to define the right scope and architecture for its companion software. Our team prepares a feature list, data flow models, and integration plan, selects the tech stack, and estimates development time and costs. You get a clear roadmap and risk-aware plan before any code is written.
Software development from scratch
We design, build, and test full-scale companion software for cardiology devices, including back-end systems, web or mobile interfaces, and integrations. Our teams cover everything from infrastructure setup and database design to device data processing, API development, and validation-ready documentation.
Low-code development
We use platforms like Microsoft Power Apps to quickly build non-regulated application modules that support cardiology product operations: internal service workflows, inventory tracking add-ons, or administrative dashboards. We handle UI design, logic configuration, system integration, and testing to ensure fast delivery without compromising security or interoperability.
Software product support and evolution
Our teams can maintain, modernize, or evolve your already deployed software solutions. This includes fixing issues, updating third-party components, improving performance, and keeping documentation aligned with regulatory standards. We can also add new features as your product evolves or new use cases emerge.
How ScienceSoft Tackles the Challenges of Medical Device Software Engineering for Cardiology
Bloating scopes and late ROI
It’s common for clients to come into a project with a large wish list. Stakeholders often want to cover every use case upfront, from complex analytics to advanced alerting, integrations, and multiple user roles. If core features are not prioritized early, this scope overload can lead to a drawn-out timeline, unpredictable costs, and long delays before the first version delivers value.
Solution
Integrating with diverse hospital ecosystems without delaying adoption
OEMs building platforms for cardiology devices often face the challenge of integrating their software into each hospital's unique IT environment. Without proper integration planning, this creates friction for provider customers who do not see how the OEM’s product can be smoothly and quickly onboarded alongside their existing tools.
Solution
Scaling device onboarding and lifecycle management without support overload
Even when data processing architecture is strong, remote monitoring products often struggle at scale due to operational friction: device provisioning, patient pairing, replacements, connectivity troubleshooting, and version fragmentation across customers. As a result, OEM teams face rising support costs and inconsistent device behavior across sites.
Solution
Reducing alert fatigue while keeping sensitivity clinically acceptable
In cardiac monitoring, “more alerts” rarely means “more value.” If thresholds are not tuned to clinical reality, alerts become noisy, clinicians lose trust, and adoption drops, even when the underlying algorithms are strong. OEMs also need to prove how alerts are generated, routed, acknowledged, and improved over time without introducing uncontrolled changes.
Solution
Managing compliance and change control throughout the project lifecycle
Software that accompanies cardiology devices often operates in a regulated context: it may fall within the SaMD scope or be tightly coupled to regulated hardware. Even minor changes to features or workflows can trigger revalidation, regulatory review, or stricter audit requirements if compliance isn’t built into the process.
Solution
Turning device data into evidence without breaking traceability and governance
Cardiology OEMs often need more than patient monitoring: they must validate algorithms, investigate edge cases, support clinical studies, and respond to post-market signals. Without a structured way to trace insights back to raw ECG segments, teams lose time, evidence becomes hard to defend, and changes to models or thresholds become risky.
Solution
Making AI features useful without adding risk or cost overhead
OEMs increasingly want to add AI features to their platforms — to improve efficiency, reduce errors, or personalize user experience. But building large, opaque models that reason on clinical data introduces compliance, cost, and explainability concerns, especially in hospital environments.
Solution
Technologies We Use to Build Software to Accompany Medical Devices
Device connectivity
Cloud services
Real-time data streaming

Data lakes
Containerization tools
Orchestration










