Cardiovascular Information Systems (CVIS)
Functionality, Integrations, and Costs
In healthcare software engineering since 2005, ScienceSoft builds CVIS solutions that boost study reader throughput, ensure consistency of reporting, and meet physicians’ expectations for performance and usability. We design integration-first systems that work seamlessly with enterprise EHR, PACS, and device fleet and support data submission to cardiology registries.
CVIS Overview
Cardiovascular information systems (CVIS) centralize key cardiology workflows, from test orders and scheduling to study interpretation and result sharing. A modern CVIS should support multi-modality imaging (echo, vascular, nuclear, CT, and MR studies), test result reporting with auto-populated measurements, ECG acquisition, hemodynamics data capture, and submission of registry-ready data for ACC programs with tight EHR and PACS integration.
ScienceSoft implements full-scale custom CVIS solutions and targeted CVIS modules or extensions for cardiovascular providers who find out-of-the-box CVIS options insufficient for their workflows.
- Ambulatory cardiology physician groups (independent or system-owned) commission lean echo and ECG domains embedded into their EHR to centralize echo reading and ECG over-read across sites. This way, they avoid implementing a costly hospital-oriented vendor CVIS that would sit outside their EHR workflows.
- Specialty cardiac hospitals commission targeted CVIS extensions that normalize structured reporting, automate NCDR/TVT registries, or tighten implant charging where their standard CVIS leaves gaps. Some cardiac hospitals also choose to replace their cardiology stack with a full-scale custom CVIS platform when their existing CVIS fails to provide service-line-wide governance and analytics.
- Medical centers and integrated delivery networks with cardiovascular service lines may deploy a custom CVIS governance layer on top of existing PACS and departmental CVIS to standardize templates, registries, and KPIs. It unifies governance, which vendor CVIS packages often fail to do.
Implementation time: 6–12+ months for a CVIS extension and 18–24+ months to engineer a full-scale CVIS.
Development costs: from $250,000 for a basic CVIS extension pack, which enhances structured reporting, registry dataset validation, and viewer performance, to $3,500,000+ for an advanced full-scale CVIS platform with an invasive cardiology module and AI automation.
Essential and Advanced Capabilities of a Cardiovascular Information System (CVIS)
Below, ScienceSoft’s consultants share a comprehensive map of CVIS capabilities that reflects the needs we most often see across our cardiology clients. In real-world projects, providers typically select and phase in only the modules that match their clinical priorities and budget to control TCO and ensure a clear payback.
Clinical workflows
Service-line management
AI in CVIS: High-Impact Use Cases
ScienceSoft embeds explainable, controlled AI modules into CVIS solutions to reduce reporting burden, boost cardiology lab throughput, and cut avoidable invasive tests without forcing clinicians to leave their enterprise EHR (e.g., Epic or Oracle Health). All AI outputs stay limited to transparent, audited suggestions protected by operational guardrails at every step, so cardiologists retain full decision-making authority.
Below are the high-impact use cases, with their clinical and operational benefits confirmed by both ScienceSoft’s clients and independent sources.
AI-assisted echocardiography
- AI-guided view acquisition with on-screen prompts and automatic capture of required echocardiography views.
- Automatic view recognition and contouring, including apical view detection, endocardial borders placement, and speckle tracking to prefill GLS measurements.
- Prefilling CVIS echocardiography report templates with physician-validated measurements.
Reported benefits: GLS measurement time cut by ~60%; reduced user-related variability.
AI-driven ECG triage for low ejection fraction
- Low ejection fraction risk scoring on routine 12-lead ECGs, flagging likely low-EF cases with calibrated scores for cardiology follow-up.
- Triggering reflex tests (e.g., echocardiography, cardiology consults, or other standardized downstream steps) for high-risk patients.
- Context-aware ECG result presentation in EHR views with flags and links to representative abnormal waveform segments.
Reported benefits: higher diagnostic yield without added echocardiography volume with 34.2% predictive value; cost-effectiveness in outpatient settings.
AI-assisted coronary CT
- Lesion-level FFR-CT mapping engine computes noninvasive fractional flow reserve (FFR) values along coronary vessels for functional stenosis assessment.
- Co-registered FFR-CT visualization overlays color-coded FFR values and confidence scores on coronary CT images for review.
- FFR-based clinical triage rules support decisions on medical therapy, invasive angiography, or revascularization for coronary lesions.
Reported benefits: threefold shorter procedure time and similar diagnostic accuracy compared to invasive FFR; ~61% fewer invasive angiographies needed.
Predictive hemodynamic management
- Predictive hypotension alerting engine scores near-term risk from arterial pressure waveforms to prompt earlier correction.
- Protocol-aware treatment guidance module suggests fluid and vasoactive dose adjustments according to site-specific protocols.
- Episode-to-report prefill service summarizes hypotensive episodes and interventions into structured entries in EHR and CVIS.
Reported benefits: 41% shorter ICU length of stay; up to a 75% reduction of intraoperative hypotension time.
AI-powered cardiology registry (NCDR) data capture
- NCDR field prefill tool extracts values from EHR notes and CVIS data and populates registry fields with confidence and coverage scores.
- Source-linked provenance layer links each suggestion to its source sentence or measurement.
- Reviewer queues with model self-learning route low-confidence items for human validation and feed corrections back to models.
Reported benefits: a ~50% reduction in registry abstraction time & cost and 98–99% inter-rater reliability (ACC).
Whether ScienceSoft develops proprietary AI modules or embeds approved third-party models into CVIS, providers still work within the same core regulatory guardrails: HIPAA or GDPR privacy safeguards, Cures Act EHI access requirements, and, where applicable, ONC HTI-1 Decision Support Interventions transparency and FDA Clinical Decision Support expectations.
We focus on building the AI integration layer with access controls, data minimization, audit trails, and clear on-screen AI disclosures aligned with recognized risk management frameworks such as the NIST AI RMF. This approach helps our clients reuse the AI vendors’ regulatory documentation and keep the additional compliance effort as light as possible.
Essential Integrations for Cardiovascular Information System
ScienceSoft recommends planning CVIS integrations up front: failing to do this early often affects software delivery speed, clinician adoption, and long-term support costs. Here are the three main decisions you will need to make:
1. Which standard data exchange protocols, versions, and identifiers should all connected systems use so that EHR, PACS/VNA, or device upgrades don’t break integrations?
The best practice is usually:
- HL7 v2 and/or FHIR for test orders, reports, and APIs.
- DICOM for images and waveforms, DICOMweb for their web viewing.
- Standard medical code sets, such as LOINC or SNOMED, for consistent analytics.
- SSO via SAML or OAuth2/OIDC, preferably SMART-on-FHIR compatible, for authorization.
2. Should clinicians access CVIS via an embedded view in the EHR or via a new-window CVIS launch?
- EHR-embedded CVIS keeps clinicians in a single UI, preserves patient and study context, shortens training, and boosts adoption, but at the cost of tighter viewer and security constraints and closer alignment with the EHR release cadence.
- New-window CVIS launch decouples EHR and CVIS upgrades and enables complex multi-monitor layouts, but demands strict patient context management.
3. Should CVIS run under enterprise imaging (enterprise PACS, VNA, and a universal viewer) or as a standalone departmental CVIS?
- Enterprise imaging CVIS (within enterprise PACS, VNA, and universal viewer) centralizes lifecycle controls for prior exams and studies, eases cross-service access, and consolidates storage and licensing under a single governance model. However, it slows cardiology feature velocity and ties releases to enterprise roadmaps and standards.
- Departmental CVIS accelerates cardiology-specific UX and structured-reporting changes, and allows the service line to control upgrades. At the same time, it risks creating a new silo, duplicate storage, and inconsistent priors unless you define hard rules for handoff to the enterprise archive and viewer.
With these decisions locked, the essential CVIS integrations below become predictable and testable instead of fragile and ad hoc:
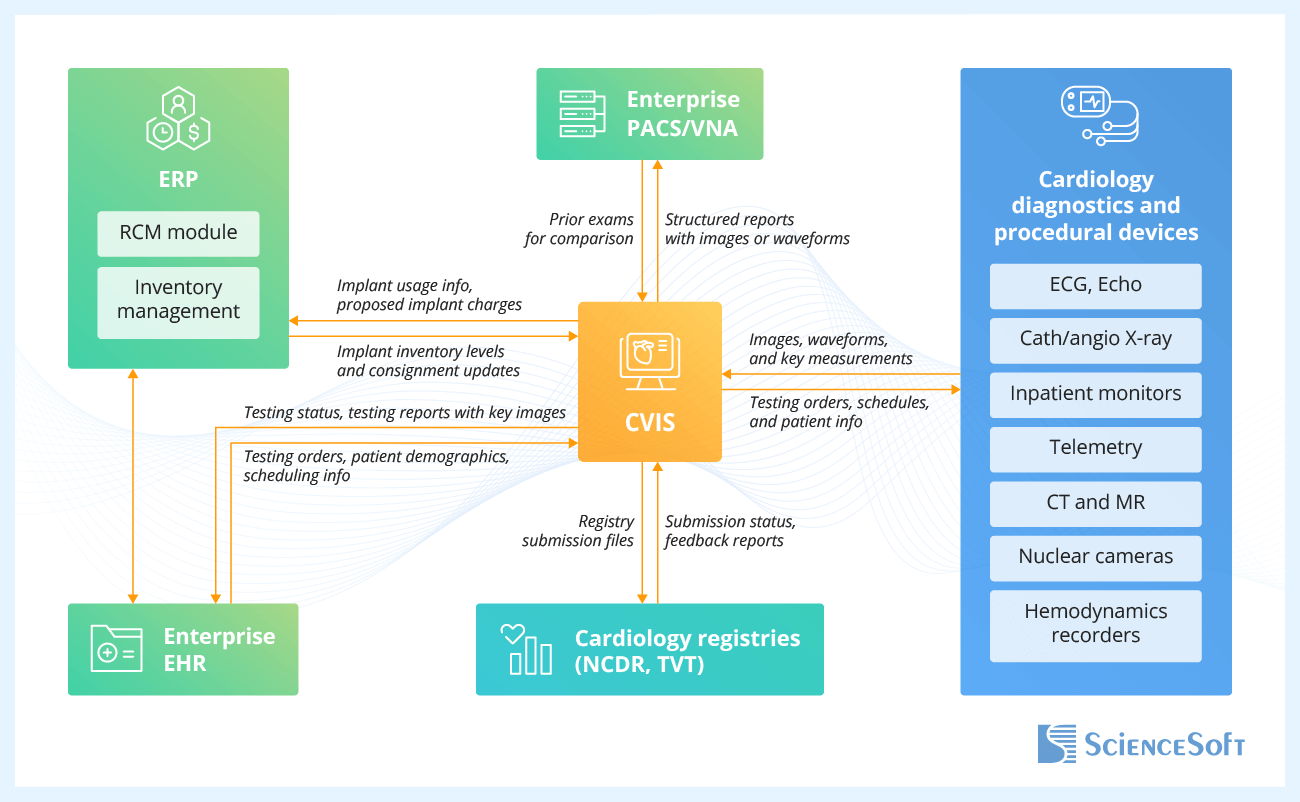
- Enterprise EHR — to anchor cardiology orders, scheduling, patient context, and testing results in one place; to spare physicians from re-entry and tab switching by allowing them to open the correct study in the CVIS viewer through single sign-on.
- Enterprise PACS and VNA — to enable enterprise-wide, zero-footprint web access to cardiology images and waveforms and their evaluation next to noncardiac studies; to unify test result storage and lifecycle, so prior exams are always available for comparison.
- Cardiology diagnostics and procedural devices — to stream synchronized images, waveforms, and measurements from diagnostic systems and patient monitors into structured reports in real time; to automatically deliver the scheduled exams and patient demographics from the order system to the imaging device.
- ERP (RCM and inventory management modules) — to feed validated implant usage, UDIs, and proposed charges from CVIS into billing workflows; to update implant inventory levels, consignment balances, and item pricing in real time.
- Cardiology registries (NCDR, TVT) — to submit registry harvest files to the registry portal and track the submission status; to import the institutional outcomes and benchmark reports for service-line quality improvement.
Field-Tested Recommendations for CVIS Development
During CVIS design, ScienceSoft recommends focusing on three key areas that often make or break real-world initiatives: structured reporting governance, predictable user experience, and compliance by design.
Structured report governance
- Structured report templates are most effective when they are governed and versioned centrally, with shared terminology and measurement lists across modalities and sites. This setup prevents label drift later and keeps downstream analytics and registry extracts consistent without manual relabeling.
- It also proves helpful when the report finalization workflow includes built-in checks for missing required fields, implausible values, and conflicting findings. In production, this typically means fewer late corrections and a higher acceptance rate on the first registry submission pass.
Predictable physician experience in the CVIS viewer
- Physician adoption usually goes more smoothly when CVIS performance expectations are made explicit and measurable rather than left at the level of “it feels slow.” Many projects benefit from defining a small set of reader-visible metrics, such as time to first image and time to open priors, and tracking them during development and rollout.
- Reader productivity also tends to improve when prior study prefetch, layout persistence, and smart loading of the most likely comparison exams are built into the design. In busy cath and echo labs, this often translates into shorter serial review sessions and less click fatigue at peak times.
Security and compliance by design
- CVIS initiatives are generally easier to defend in audits when the design phase already covers how electronic health information flows out of the system: to patient portals, to other clinicians, to registries, and to HIEs under the 21st Century Cures Act. Making these paths explicit during development reduces surprises later.
- Accounting for HIPAA Security Rule requirements during CVIS architecture design usually means less rework later. It forces early decisions on access control, audit logging, and encryption that fit actual cardiology workflows and data flows, rather than retrofitting controls onto processes after they are already defined.
How Much Does It Cost to Develop a CVIS?
In ScienceSoft’s experience, the cost of developing a CVIS typically ranges from $250,000 for focused extensions to over $3,500,000 for a full-scale platform. Project budgets largely depend on the number and complexity of device, EHR, and PACS integrations, invasive cardiology scope, and AI automation (registry data abstraction, image quantification add-ons, ECG triage, and more).
The table below outlines feature bundles and indicative costs for the most common CVIS implementation scenarios.
|
|
Standard functionality |
Advanced functionality |
|---|---|---|
|
CVIS extension pack
?
For specialty cardiac hospitals needing to fix critical CVIS gaps without replacing their existing platform. |
Cost: $250,000–$650,000 |
Cost: $650,000–$1,200,000+ |
|
Echo and ECG domain build
?
For multi-site ambulatory cardiology groups that prioritize unified echo and ECG domains and tight integration with their EHR. |
Cost: $450,000–$900,000 |
Cost: $900,000–$1,400,000+ |
|
Enterprise imaging convergence or migration
?
For IDNs that need to align cardiology imaging with enterprise VNA, universal viewer, and standardized analytics. |
Cost: $700,000–$1,400,000 |
Cost: $1,500,000–$2,400,000 |
|
Full-scale CVIS build
?
For specialty cardiac hospitals aiming to consolidate imaging, ECG, hemodynamics, and registries into a single enterprise CVIS. |
Cost: $1,400,000–$2,500,000+ |
Cost: $2,400,000–$3,500,000+ |
Why Choose ScienceSoft as Your CVIS Development Partner
- Since 2005 in healthcare software engineering and IT consulting.
- 150+ successful projects in the domain.
- Since 2012 in image analysis; since 1989 in data analytics and AI.
- Architecture and Solutions CoE with HIMSS-certified specialists to design secure and highly integrated CVIS even in legacy-heavy environments; in-house PMO to steer the project to success despite budget constraints and diverse clinician expectations.
- Proficiency in aligning healthcare software with HIPAA, GDPR, the 21st Century Cures Act, ONC Cures Final Rule, CEHRT, ONC HTI-1 Decision Support Interventions transparency rules, and FDA Clinical Decision Support guidance.
- Expertise in imaging and data exchange standards, including DICOM and DICOMweb, SCP-ECG, HL7 v2, FHIR, IHE XDS/XDS-I, C-CDA, USCDI, and SMART on FHIR; clinical coding and terminology standards, such as ICD-10-CM, CPT, SNOMED CT, and LOINC, and quality/registry formats QRDA and NCDR. HL7® FHIR® certified implementers on the team.
Our awards, recognitions, and certifications
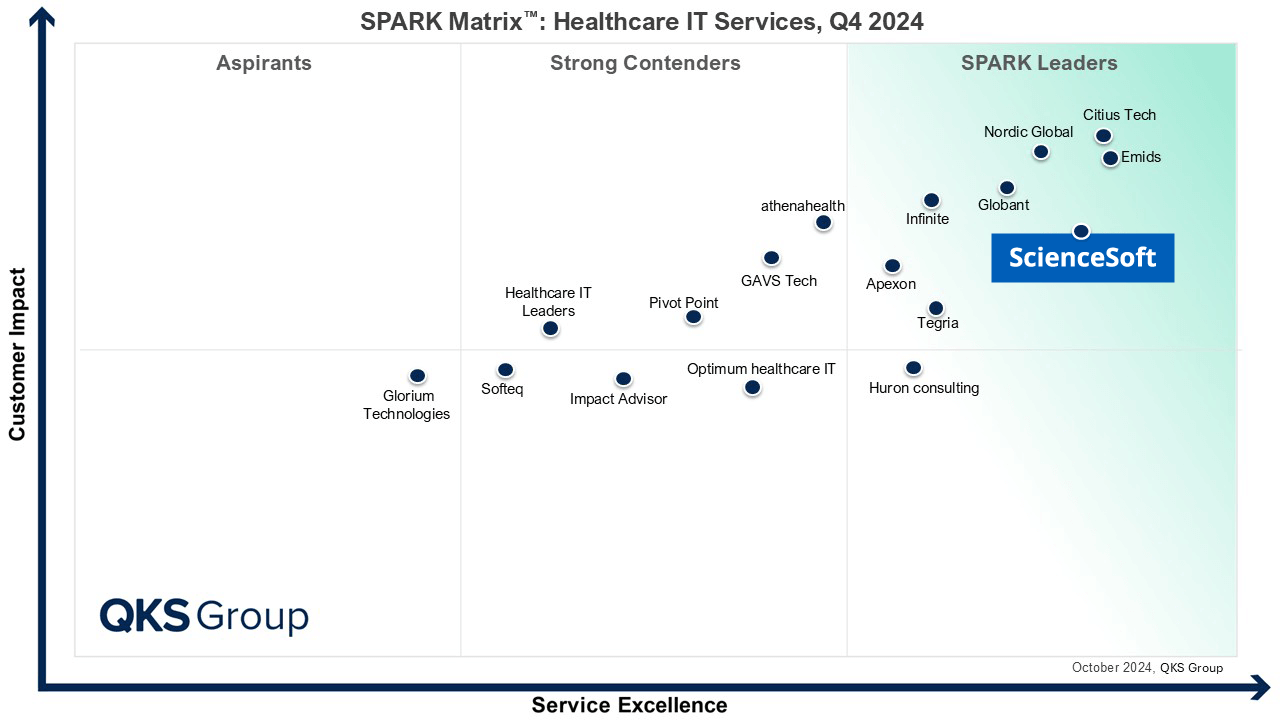
Featured among Healthcare IT Services Leaders in the 2022 and 2024 SPARK Matrix
Recognized for Healthcare Technology Leadership by Frost & Sullivan in 2023 and 2025
Named among America’s Fastest-Growing Companies by Financial Times, 4 years in a row

Top Healthcare IT Developer and Advisor by Black Book™ survey 2023
Four-time finalist across HTN Awards programs

Named to The Healthcare Technology Report’s Top 25 Healthcare Software Companies of 2025

HIMSS Gold member advancing digital healthcare
ISO 13485-certified quality management system
ISO 27001-certified security management system




