EHR for Oncology
Specialized Functionality, AI Enhancements, and Costs
In healthcare IT since 2005, ScienceSoft designs secure EHR solutions that efficiently automate oncology workflows. Our in-house MD consultants guarantee software compliance with HIPAA, GDPR, Cures Act, HITRUST, 45 CFR Part 170, 21 CFR Part 11, and other necessary regulations.
A Brief Look at EHR in Oncology
EHR systems for oncology securely store medical records of cancer patients, supporting specialized diagnostics, treatment planning, chemo- and radiotherapy tracking, and survivorship care processes. When dealing with out-of-the-box EHR systems, clinicians typically report friction with documenting chemotherapy regimens, capturing staging and biomarker data in structured fields, coordinating care across medical, surgical, and radiation oncology workflows, and meeting demanding reporting requirements for registries and value-based care programs. Limited interoperability makes it harder to exchange genomic results, treatment histories, and outcomes data across networks.
Oncology clinics opt for custom solutions when they need to streamline regimen management, automate staging and reporting, and make oncology-specific clinical data immediately usable for care coordination, analytics, and compliance.
- Useful integrations for oncology EHR software: RIS, LIS, NGS LIMS, PACS, Digital Pathology, ROIS/TPS, pharmacy networks, HIE, RPM, CTMS, patient portal/app, cancer registries, payor systems.
- Implementation time: 12 to 24+ months.
- Development costs: $400,000–$2,000,000+. Use our free calculator to estimate the cost for your case.
EHR Capabilities Tailored for Oncology
How AI Can Enhance Oncology EHR Workflows
Artificial intelligence in EHR systems is often used to reduce administrative burden and streamline clinical processes. Below is a list of the most likely AI use cases for oncology workflows.
Administrative support
- Speech recognition to enable voice control, dictation, and patient-doctor conversation transcription.
- Extracting data from disparate sources (e.g., lab tests, clinical notes) to structure and categorize it in EHR.
- Smart search entry suggestions and info prioritization based on the context and semantics.
- Chatbots for patient scheduling, informational support, etc.
Clinical decision support (AI module may require regulatory clearance)
- Patient record analysis (across lab results, imaging assessments, therapy history, etc.) to detect patterns, trends, and abnormalities, identify high-risk patients, and catch deterioration early.
- Predictive analytics to forecast response to treatment, toxicity levels, survival rates, and more.
- Guideline-based treatment recommendations based on structured cancer data (e.g., stage and biomarkers) or genomics results.
- AI-assisted drug dosage calculation and medication reconciliation for multi-drug chemotherapy regimens.
- Radiology and pathology image analysis.
Valuable Integrations for an Oncology EHR System
To unlock the full support of modern cancer care, ScienceSoft recommends integrating the EHR with the following systems:
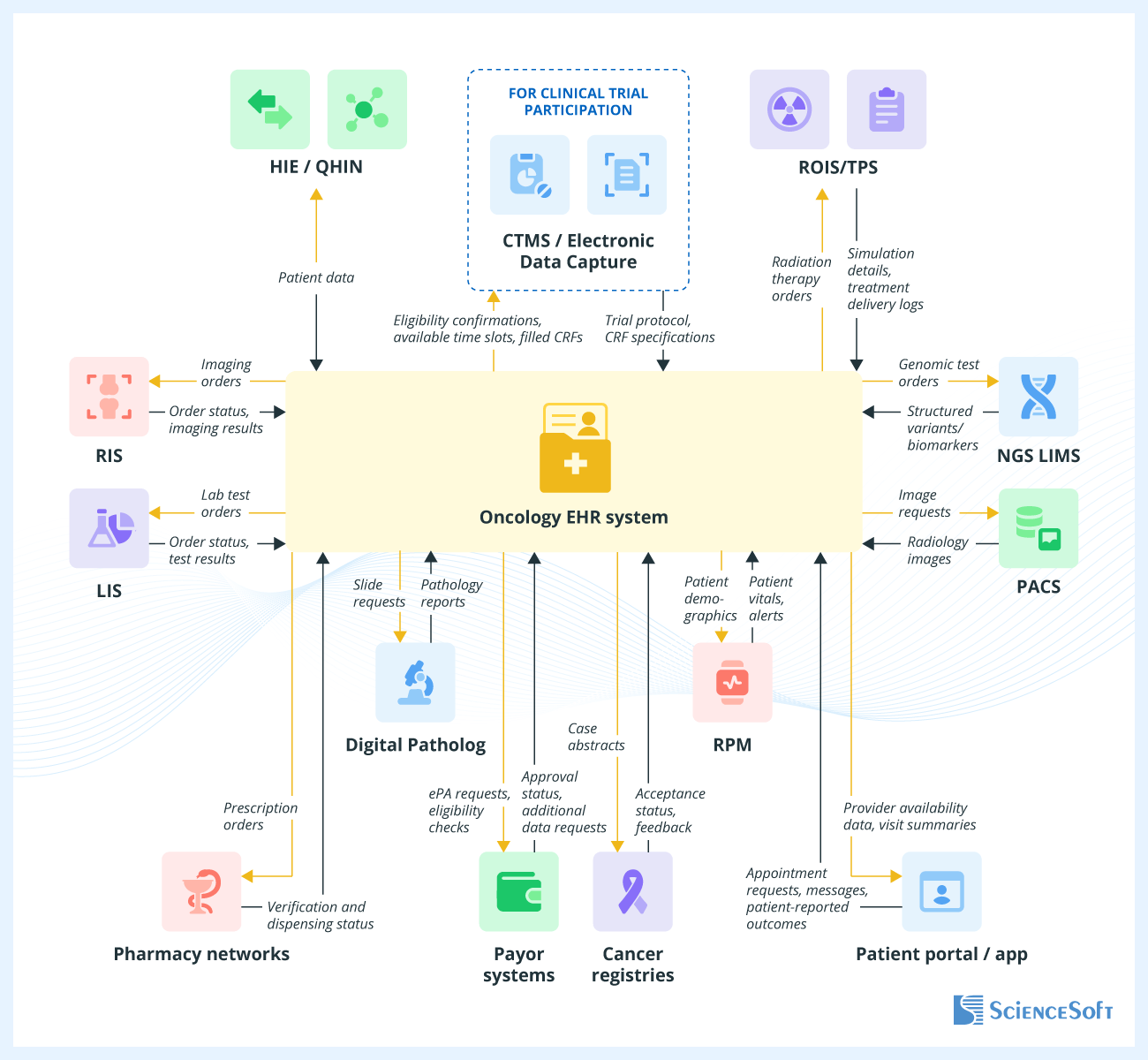
- Health information exchange (HIE) software to securely transmit structured oncology data between providers (e.g., diagnostic laboratories, primary care practices).
- Radiology information system (RIS) to manage imaging orders, schedule patients for CT, MRI, and PET scans, and view results directly in electronic health records.
- Laboratory information system (LIS) to schedule patients for pathology, genomic, or biomarker tests and integrate the results directly into patient medical histories.
- NGS laboratory information management system to manage tumor/germline genomic test workflows and return structured variants and biomarkers directly into the patient record for decision support and trial eligibility.
- Picture archiving and communication system (PACS) for immediate access to radiology images.
- Digital pathology system to request whole-slide imaging and synoptic pathology reporting, and to integrate CAP checklist fields (margins, grade, biomarkers) as discrete data that feed staging and treatment planning.
- Radiation oncology information system / treatment planning system (ROIS/TPS) to coordinate CT simulation, radiation therapy planning, and delivery.
- Pharmacy networks to send electronic prescriptions directly to the pharmacy and track verification and dispensing status.
- Payor systems to verify coverage and benefits in real time and to submit, track, and manage electronic prior authorizations for imaging, radiation therapy, and specialty drugs, as well as exchange claims and remittances via HIPAA X12 837/835 when claims processing is included.
- Remote patient monitoring (RPM) to track post-treatment or between-cycle patient status via connected devices.
- Cancer registries to streamline compliance and quality reporting.
- Clinical trial management system / electronic data capture (CTMS/EDC) to automate clinical trial matching, schedule and monitor trial-related visits, lab tests, and imaging, and streamline reporting.
- Patient portal or app to enable appointment scheduling, easy access to visit history and clinical summaries for patients (including CCDA-based clinical summary downloads), personalized lifestyle recommendations, etc.
How to Build an EMR System for Oncology
1.
Discovery and project planning
- Analyze current administrative, billing, and cancer care-specific clinical workflows.
- Engage key stakeholders (oncologists, radiation therapists, nurses, admin staff, patient advocates, and tumor board specialists) to collect their feedback on the limitations of the current processes.
- Translate the business needs into functional and non-functional requirements. Document them in a software requirements specification (SRS).
- Identify relevant compliance requirements (e.g., HIPAA, GDPR, 45 CFR Part 170, 21st Century Cures Act) and plan for required registration processes (e.g., ONC certification, FDA/MDR clearance for SaMD functionality).
- Work out a migration strategy for the existing system's data.
- Create the project roadmap, specifying the required talents, timeframes, budget, and KPIs.
2.
System design
- Outline the logical and functional components of the system-to-be, define their interactions, and create detailed architectural diagrams.
- Plan for integrations with the necessary internal systems (e.g., LIS, PACS, ROIS/TPS, HIE/QHIN endpoints) using appropriate standards (FHIR/USCDI, HL7 v2/v3, XDS/XDS-I).
- Select a technology stack that aligns with the functional and non-functional requirements and regulatory needs of the system.
- Create user personas and map out their individual journeys (adding a new patient profile, building a treatment regimen, creating a prescription order, etc.). Then outline how primary clinical processes move across different roles (e.g., for medical oncology: oncologist → pharmacy → nurse; and for radiation oncology: dosimetrist → physicist → radiation oncologist)
- Design UX wireframes and conduct usability testing.
- Build UI prototypes based on the validated wireframes.
For years, oncology data was scattered across hospitals in free-text notes, PDFs, and siloed specialty systems, making it hard to share or reuse. Only recently have U.S. government entities begun to recognize this gap, leading to a wave of new interoperability initiatives. USCDI+ Cancer defines a nationally recognized set of data elements so that cancer-related information is captured consistently. Next, mCODE (Minimal Common Oncology Data Elements) is a FHIR Implementation Guide that provides standard “vocabulary” and structure for key oncology concepts (e.g., diagnosis, TNM staging, line of therapy, and outcomes) so that everyone records them the same way. Finally, FHIR Genomics adds a structured format for storing and exchanging genomic test results, including specific gene variants, multi-gene panels, and the clinical interpretations that guide targeted therapies. Together, these standards create a common language for oncology care, making it far easier for EHRs, research systems, and registries to work with your data without building custom connections.
3.
Iterative development and testing
- Develop the system’s front end and back end in short, feedback-driven iterations.
- Implement and test integrations with the required internal software.
- Implement relevant security measures: multifactor authentication, role-based consent-aware access, audit logging, data encryption, and identity proofing.
- Carry out functional (including clinical simulation), integration, performance, security, compliance, compatibility, and accessibility testing.
4.
Pre-launch activities
- Update and finalize software documentation.
- Conduct internal audits on compliance with HIPAA, EPCS, GDPR, the 21st Century Cures Act, and other applicable regulations.
- Migrate structured and unstructured data from legacy systems.
- Create training materials for oncology staff.
- (Optional) Submit the EHR for ONC certification and FDA clearance for SaMD components.
5.
Deployment and monitoring
- Deploy the EHR system to the production environment and validate stability.
- Implement a structured onboarding and training plan for all user groups across oncology service lines.
- Establish a 24/7 help desk and troubleshooting teams.
- Track adoption and collect feedback among oncologists, nurses, and administrative users.
- Monitor the industry for standard, guideline, or regulation updates and perform the necessary system modifications.
Technologies ScienceSoft Uses to Build Secure EHR/EMR Software
Low-code development
Device connectivity
Machine learning platforms and services
AWS





Azure
Blockchain frameworks
Telehealth
Video streaming
Messaging and conferencing
Payment gates

EHR for Oncology Development Costs
The cost of building an EHR system for oncology can vary from $400,000 to $2,000,000+, depending largely on functional scope and required integrations.
From $400,000
For a system that covers core medical oncology workflows (regimen building, dose calculation, e-prescribing, etc) and minimal clinical and operational data visualization.
From $800,000
For software that introduces radiation therapy capabilities (record-and-verify, image fusion, contouring tools, etc.) and streamlines compliance reporting.
From $2,000,000
For an enterprise-level system that combines medical and radiation oncology capabilities with clinical trial support, patient engagement tools, and advanced financial and operational analytics.






