Digital Therapeutics Software
Features, Costs, and Gains
Since 2005 in the development of healthcare IT solutions, ScienceSoft offers comprehensive digital therapeutics consultancy and implementation services.
Digital Therapeutics: the Essence
Digital therapeutics (DTx) aims at addressing health conditions using specifically designed and clinically tested technologies. Digital therapy solutions provide the functionality to:
Prevent diseases
For example, apps for patients with prediabetes.
Manage diseases
Among the common examples are an asthma management system that monitors medication adherence and identifies asthma triggers, and AI-based coaches to treat mental health conditions.
Custom digital therapeutics software can help handle a specific condition according to a dedicated tech-enabled therapy program. DTx apps bring value to patients who use them by providing access to unique and research-backed health management tools.
Architecture of a Digital Therapy Solution
Digital therapy products vary from standalone apps to complex solutions that include patient-facing software and connected medical devices.
Here’s a sample architecture of a digital therapeutics platform with a doctor-facing app and a patient-facing app that targets patients with diabetes:
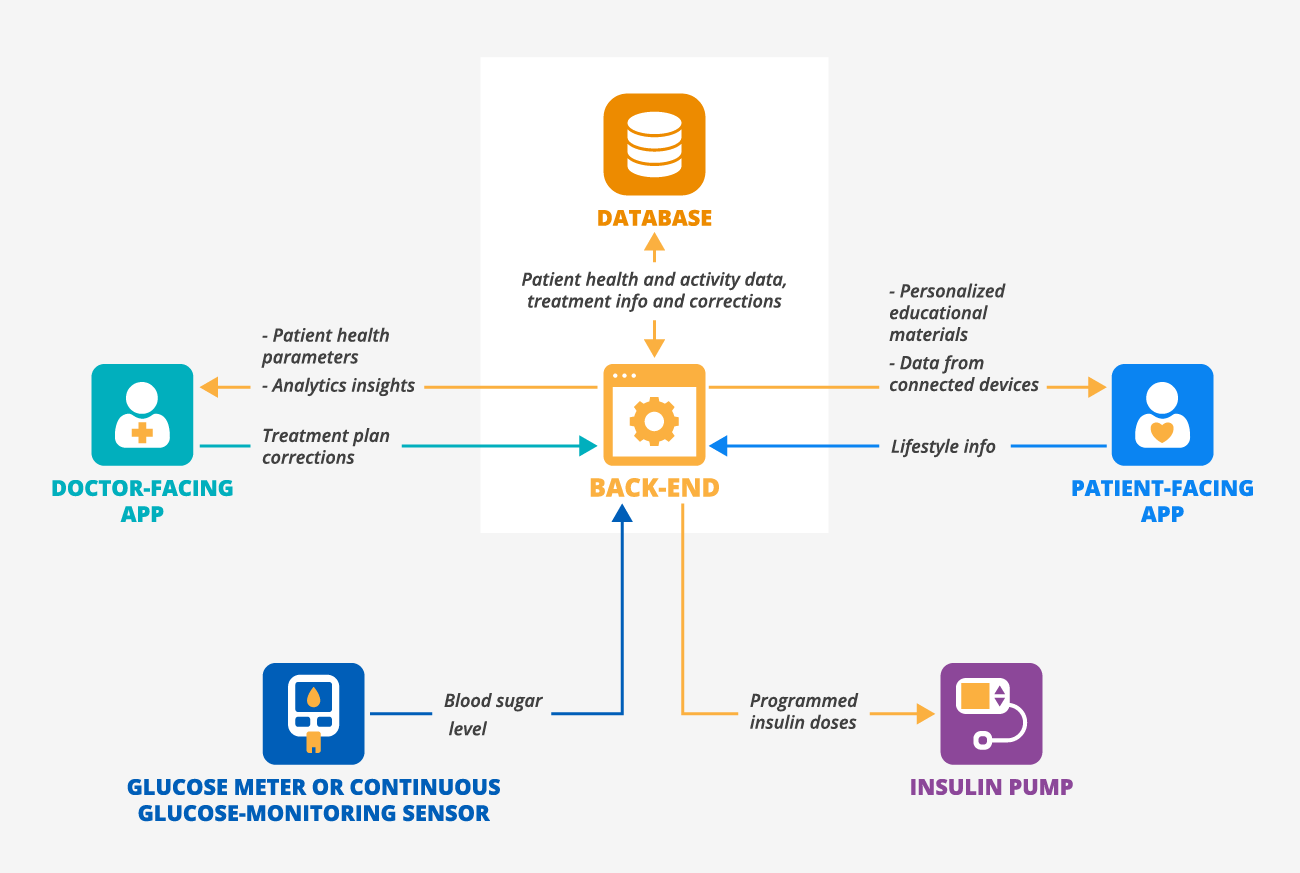
Doctor-facing app contains:
- List of patients with diabetes and their recent vitals (blood glucose, insulin intake, HbA1C).
- Notifications on abnormal health parameters.
- Treatment plan correction (basal insulin doses, diet, etc.).
- Lifestyle info and blood tests correlation analytics.
Patient-facing app contains:
- Reminders to log parameters (daily activity, exercise, diet).
- Bolus insulin calculator and doses programming.
- Chatbot to learn more about diabetes, low-sugar diet, etc.
- Blood glucose level and insulin tracking.
Key Features of Digital Therapeutics Solutions
Based on ScienceSoft's experience in digital therapeutics software, our healthcare IT consultants made a list of the key features of such solutions.
Treatment adherence monitoring
- Medication intake reminders for patients.
- Reminders for patients to log health parameters.
- Abnormal health parameters alerts for a patient and a doctor.
- Personalized health recommendations for patients (to exercise, to follow a specific diet, etc.).
Health data collection
- Automation of health data entry from connected devices.
- Manual or voice recognition-powered health data entry and editing for patients.
- Lifestyle data entry (exercise, sleep, diet, water intake) by a patient.
Disease management for patients
- Virtual AI-based coaches that guide patients through recovery programs.
- Video guides (breathing exercises, panic attacks overcoming techniques, etc.)
- Patient-facing AI chatbots helping drive lifestyle changes (e.g., for cognitive and behavioral disorders, substance abuse).
- Therapy technologies (video-based reminiscence therapy, gamified attention-improving therapy, etc.).
Doctor-facing analytics
- Patients’ health parameters reports and trends visualization.
- ML-driven suggestions on treatment modifications.
- Drug uptake analytics to adjust the care plan.
Patient-doctor interactions
- Chats for quick communication between a doctor and a patient.
- Telehealth functionality for remote digital health support.
- E-visit recordings history.
Security
- Regulatory compliance (e.g., with HIPAA, FDA).
- Password-protected access through healthcare organization-owned or patient-owned devices.
How to Ensure Compliance of DTx Software
To qualify as a digital therapeutics solution, the software should:
- Incorporate patient privacy and data security practices.
- Be supported by published trial results in peer-reviewed journals.
- Receiving clearance or certification by relevant regulatory bodies.
When DTx software performs medical purposes (e.g., disease management or treatment) and directly influences a patient's health, it may be considered software as a medical device (SaMD). To ensure software safety, SaMD should be submitted to the FDA or other relevant agencies (for non-US apps) for review and approval.
Among the key approval factors for the authorities is clinical efficiency for the targeted condition. ScienceSoft is ready to help you develop your DTx app and prepare sufficient documentation for FDA submission or CE marking.
Popular Integrations for Digital Therapeutics Apps
Although DTx forms vary, they all need integrations with healthcare software and hardware to coordinate patient treatment, assess disease management progress, and more. To promote interoperability throughout the IT ecosystem, it's useful to adopt standardized APIs (e.g., FHIR), datasets (e.g., USCDI), and clinical terminologies (e.g., LOINC, SNOMED CT, RxNorm). Here is the list of integrations recommended by ScienceSoft's consultants:
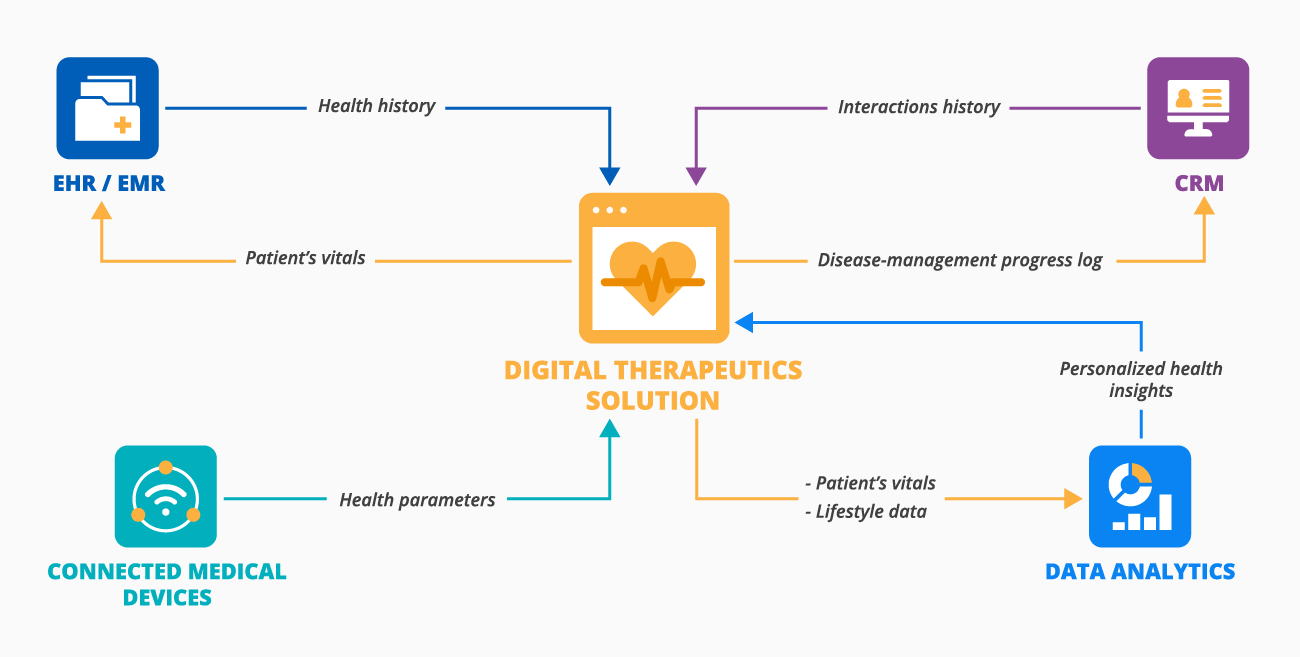
- EHR or EMR (Electronic Health Records or Electronic Medical Records) - to store patient-generated health data and exchange a patient’s health history with a DTx solution.
- CRM (Customer Relationship Management) - to adjust the treatment management according to the CRM-based interactions (e.g., recommending new educational materials) and to automatically update the patient info in CRM with the DTx solution’s data (e.g., number of digital therapy sessions).
- Connected medical devices - for automated vitals or medication intake collection from glucose meters, smart thermometers, etc.
- Data analytics software - to analyze patient-generated data, medication intake, and spot lifestyle and health parameters correlations.
How to Develop Digital Therapeutics Software
|
1. Analyzing the market, competitors, and potential users of a future DTx app to conceptualize the app and define a marketing strategy. 2. Creating a regulatory compliance roadmap, classifying the DTx app according to SaMD types, and defining the FDA, MDR, or other registration strategy. 3. Defining software requirements and features and planning the development and testing process. 4. Planning a tech stack and architecture, creating complete project documentation for further regulatory submission. |
5. Creating a PoC (proof of concept) to make sure the app is efficient for its clinical goal. 6. Developing and testing the DTx product. 7. Conducting clinical trials of the innovative DTx product on the focus groups under clinical supervision. 8. Submitting the app for registration or clearance to the relevant authorities (e.g., FDA). 9. Launching the digital therapeutics software and implementing the marketing strategy to promote the app. |
What Determines DTx Software Success
To help you stay relevant in the competitive market, ScienceSoft has listed the best practices we apply to deliver efficient digital therapeutics apps:
An early working version of the software
Agile digital therapeutics software development allows you to certify the solution and start trials at an early stage, create focus groups, and get users’ feedback on product’s usability.
Patient engagement
Similar to medicine intake, DTx solutions should be used properly and systematically to drive positive health outcomes. When developing custom digital therapeutics software, ScienceSoft implements user engagement techniques (e.g., achievement badges, challenges) to facilitate better care outcomes.
Advanced data analytics
To provide physicians with consolidated patients’ health insights, not just a list of parameters, and guarantee that DTx-supported treatment leads to a patient’s health improvements.
Investments and ROI
Digital therapeutics solutions typically cost between $300,000 and $800,000, with development investments depending on the software complexity, expected feature set, and integration with medical devices. DTx solutions have an average payback period of 1 year and can yield an average ROI of 250%.
Key financial outcomes
- Reduced time per patient with a chronic condition.
- Improved healthcare outcomes due to continuous disease management.
- Reduced hospitalizations related to chronic diseases.
- Increased care access without workforce expansion.
When Custom DTx Solution Is Your Best Choice
ScienceSoft's experts suggest that custom digital therapeutics development may be a preferred option if a healthcare organization aims to:
|
|
Cover specific digital health needs (e.g., creating a DTx platform that consolidates 2-3 disease-specific solutions). |
|
|
Cost-effectively enable multiple integrations of a DTx solution with an organization’s software ecosystem (EHR/EMR, HIS, LIS, CRM, Practice Management System, the IoMT cloud). |
|
|
Provide branded UI that can be difficult or impossible to configure in ready-made DTx products. |
DTx Solution Implementation
Holding ISO 13485 certification, ScienceSoft helps healthcare organizations design, develop and implement reliable digital solutions according to the requirements of the FDA and the Council of the European Union. Our top priority is to ensure your project's success, overcoming time and budget limitations, and adapting to evolving requirements.
Digital therapeutics product consulting
- Market analysis to design a DTx product concept, competitive advantage, etc.
-
A feature list for the DTx solution.
-
Brand positioning, a comprehensive UI kit, and interactive UI prototypes.
-
Outline of possible ways to build the DTx product.
- Timing and cost estimations.
- Regulatory compliance consulting (HIPAA, the Cures Act, GDPR, FDA, MDR, etc.).
Digital therapeutics implementation
- Digital health needs analysis, project planning, and design.
- DTx solution development and testing.
- Integration with healthcare IT systems (EHR, CRM) and devices (glucometers, insulin pumps, etc.).
- Ensured compliance with relevant regulations (HIPAA, the Cures Act, GDPR, FDA, MDR, etc.).
- A DTx solution’s after-launch support and maintenance.
About ScienceSoft
Headquartered in Texas, US, ScienceSoft has been providing quality IT services for the healthcare industry since 2005. Being ISO 13485-certified, we design and develop medical software according to the requirements of the FDA and the Council of the European Union. If you consider digital therapeutics to level up remote and inpatient care and improve health outcomes, engage our team for assistance.

