HIPAA-Compliant Software Development
A Comprehensive Guide
Since 2005, ScienceSoft has been mastering medical software development to design and implement HIPAA-compliant healthcare software.
The Essence of HIPAA-Compliant Software Development
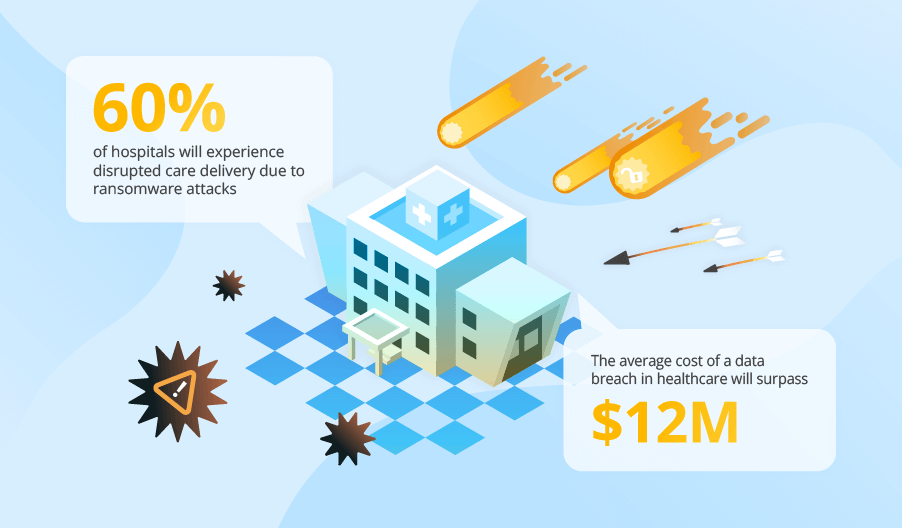
HIPAA-compliant software development is a must when creating US-targeted software that stores, processes, or transfers personal health information (PHI). ScienceSoft is ready to help you follow HIPAA rules and stay safe from such noncompliance issues as data loss or theft, legal claims, and fines.
Based on its practical expertise, ScienceSoft knows how to set up a cost-effective and reliable process of HIPAA-compliant software development.
- Time: From 4 months (e.g., for an MVP of a simple application for patient-doctor communication) to 12+ months for a full-fledged remote patient monitoring system.
- Team: A project manager, a business analyst, a regulatory consultant, a solution architect, UI and UX designers, front-end and back-end developers, an information security specialist, a QA engineer, and a DevOps engineer.
- Cost: Starts from $30,000 for a single-functionality mobile patient app without EHR integration (e.g., medication intake monitoring). Use our free calculator to take the guesswork out of your project budgeting.
FAQ
How can my organization become HIPAA-compliant?
HIPAA compliance goes beyond software development. To be fully HIPAA-compliant, you should implement administrative safeguards (e.g., risk assessment, employee training), technical safeguards (e.g., user authentication, access control), and physical safeguards (e.g., facility access, workstation security).
Do I need HIPAA certification to be compliant?
No, you don't. HIPAA doesn't have a formal certification procedure. According to the HIPAA Security Rule, you should implement the HIPAA measures and be ready for the following audit. However, there are plenty of misleading HIPAA certification proposals on the market whose certification results are not recognized by the US government.
Should a vendor involved in developing HIPAA-compliant software sign a Business Associate Agreement?
Yes. Under HIPAA, the covered entity and a business associate (in this case, a software development company) should have a written contract to ensure that a business associate will adequately protect ePHI.
HIPAA-Compliant Software Development Steps
Specifics of a HIPAA-compliant development plan depend on the type of medical software and its functional scope. ScienceSoft designed the following generalized plan of HIPAA-compliant medical software development based on our 20 years of hands-on experience in the domain.
1.
Gather compliance requirements and design compliant software
- (When you outsource any project activities) Signing of a Business Associate Agreement with a consulting or development partner having access to PHI to ensure their legal obligation to follow HIPAA software compliance guidelines on data security, encryption methods, security practices documentation, etc.
- (For product companies) Market and competitors research in the respective medical software niche, identification of target customers, software idea productization.
- (For healthcare organizations) Business needs analysis and elicitation of medical software requirements.
- Gathering compliance requirements for the HIPAA regulation and other applicable federal and local legal acts, standards, and guidelines of appropriate governmental agencies (FDA, FCC, SAMHSA, etc.).
- Listing and prioritization of medical software features, outlining main user scenarios (e.g., for patients, doctors), splitting features into subscription plans (for medical software products).
- Designing the architecture and integrations of HIPAA-compliant software, selecting a fitting tech stack, and aligning integrations with applicable standards (e.g., FHIR/USCDI, HL7 v2/v3, CCDA, XDS/XDS-I). Note: Cloud services in use should comply with HIPAA.
- (For medical software products) Planning configuration capabilities that allow users to modify certain software modules.
- Assessment of usage risks (e.g., potential software misuse) for HIPAA-compliant software and risk mitigation plan design.
Phase deliverables:
- Signed Business Associate Agreement.
- Medical software feature list with traceability between user/business needs and the features.
- Software compliance specification with references to appropriate clauses of standards and regulations (HIPAA, CCPA, etc.).
- Software requirements specification.
- A high-level design of medical software architecture.
- Medical software usage risks description and a mitigation plan.
2.
Plan a HIPAA-compliant software development project
At ScienceSoft, this step includes:
- Project scope identification.
- Choosing a development approach for HIPAA-compliant software.
- Assessment of software development risks (e.g., delays in the development).
- Budget planning.
- Project scheduling (planned development iterations, key milestones, etc.) and KPI planning.
Phase deliverables:
- Budget plan.
- Project schedule and KPI plan.
- Mitigation plan for medical software development risks.
Best practice: ScienceSoft’s project managers plan Agile development (Scrum, Kanban, etc.) for complex medical software development projects to easily accommodate potential project scope changes in later project stages.
3.
Design UX and UI for the software
- UX design – to visualize key software functionality in the identified user scenarios and plan convenient journeys for medical software users (patients, medical specialists, admins). The user journeys are planned taking into consideration HIPAA compliance and security measures like user session timeout, emergency access to patients’ data, software access control, etc.
- UI prototyping – to visualize software look for different user groups.
- UI design – to create graphic interface elements of healthcare software according to the defined visual accessibility requirements.
Phase deliverables:
- UX wireframes.
- User interface design documentation (all screens, assets, and source files).
Best practice: If a client needs to cut the healthcare software development time, ScienceSoft launches the UX and UI design stage simultaneously with the software project planning stage.
4.
Develop healthcare software
During this stage, ScienceSoft conducts:
- Back-end software development – to develop the server side of the application and APIs with a focus on PHI security.
- Front-end development of healthcare software – to transform UI design elements into a functioning user side.
- Testing – to detect and fix medical software defects, check software against its functional requirements, verify software stability, security, and HIPAA compliance, validate usability and accessibility for target users.
Phase deliverables:
- Developed software.
- Detailed architecture design.
- Application’s source code.
- Test documentation.
Best practice: ScienceSoft usually starts with creating an MVP for HIPAA-compliant software to quickly present core software functionality with a simple UI design. It allows getting early stakeholder or market feedback on software and adding new features to it based on the feedback analysis.
Having worked with healthcare providers for more than a decade, I recommend starting with MVP development. Creating HIPAA-compliant software MVP helps quickly present core software functionality with a simple UI design and allows getting early feedback on software from the stakeholders or target users. Further, you can add new features to the app based on the feedback analysis.
5.
Conduct pre-launch activities and launch the software
- Revision of HIPAA-compliant software usage risks – to identify new usage risks, address them, and update the risk mitigation plan.
- Revision of HIPAA-compliant software documentation – to check if any medical software requirements changed during the development process and update software documentation to ensure documentation cohesiveness for future HIPAA compliance audits.
- Software compliance check according to relevant medical standards (e.g., IEC 82304-1:2016 for software integrated with smart medical devices or sensors) and, where applicable, 21st Century Cures Act interoperability requirements (FHIR API conformance, USCDI data classes).
- Pilot rollout of medical software for a focus group – to present the fully developed app to the focus group (e.g., a group of patients in a hospital), evaluate their satisfaction with the app, get their feedback, and adjust software accordingly.
- Launch of HIPAA-compliant software.
Phase deliverables:
- Deployed medical software.
- Revised healthcare software documentation.
- App setup guide, admin guide, support guide, user guide (for all user groups).
6.
Maintain, audit, and modernize healthcare software
ScienceSoft offers our healthcare clients the following after-launch services:
- Medical software support and maintenance – to fix revealed defects, implement security patches, resolve incidents, and ensure uninterrupted work of medical software.
- HIPAA compliance and security audits – to regularly verify medical software and its infrastructure for HIPAA compliance, check PHI security, etc.
- Evolution of medical software – to implement new medical software features according to user feedback.
Consider Professional Services for HIPAA-Compliant Software Development
Since 2005 in medical IT, ScienceSoft offers end-to-end HIPAA-compliant software consulting and development services. Our mission is to drive your project success, navigating time and budget constraints, as well as adapting to changing requirements. You set goals, we deliver on them.
HIPAA-compliant software consulting
We offer:
- Healthcare software concept design.
- Development and launch plan.
- High-level architecture and integrations design, tech stack selection.
- HIPAA compliance checklist for your healthcare software specifics.
- Estimation of costs, ROI, a payback period.
Outsourcing of HIPAA-compliant software development
We offer:
- A concept and a feature set of HIPAA-compliant software.
- UX and UI design.
-
Healthcare software development and testing.
-
All required documents for HIPAA compliance audit and pre-audits upon the agreed schedule.
-
Maintenance, support, and evolution.
Why ScienceSoft
- Since 2005 in healthcare IT and since 2003 in cybersecurity.
- Mature quality management system confirmed by ISO 13485 and ISO 9001 certifications.
- ISO 27001 certification to guarantee clients’ data security.
- Working experience with healthcare standards (e.g., HL7 v2/v3, FHIR, CCDA, USCDI, DICOM, ICD-10, CPT, LOINC, RxNorm, XDS/XDS-I, HIPAA X12 837/835).
- A Healthcare IT Services Leader in the 2024 SPARK Matrix.
Our awards, recognitions, and certifications
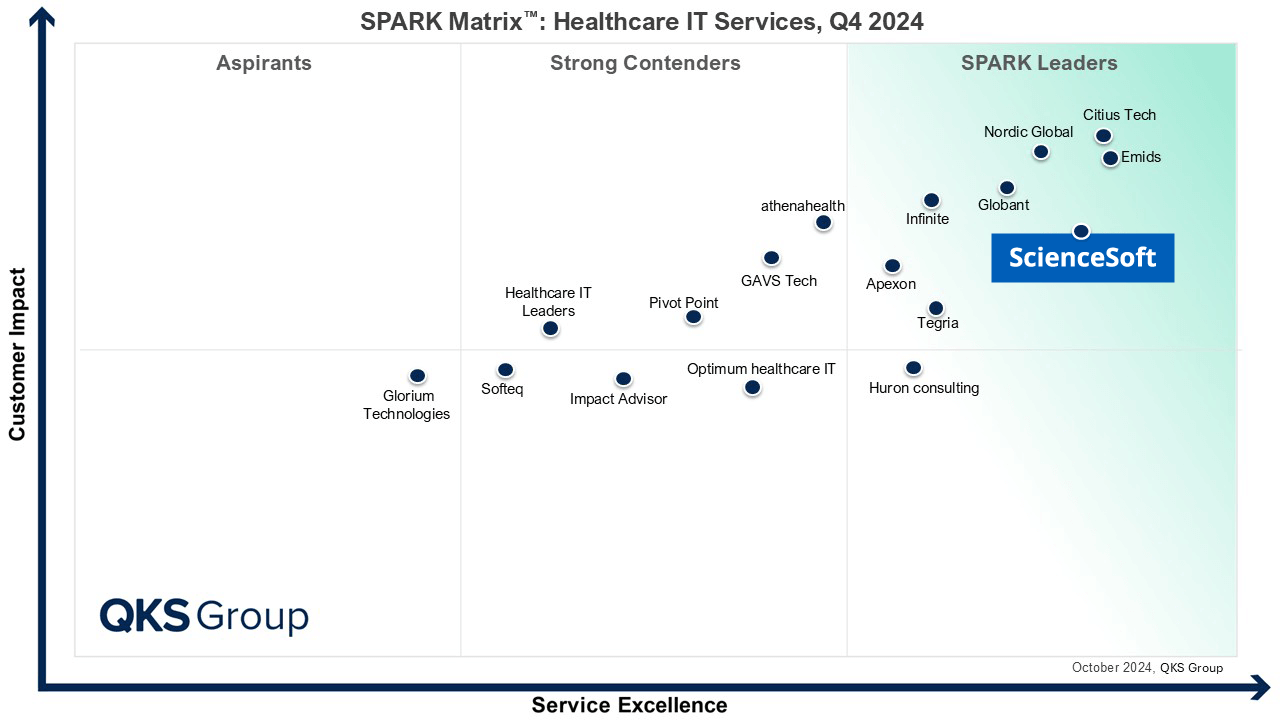
Featured among Healthcare IT Services Leaders in the 2022 and 2024 SPARK Matrix
Recognized for Healthcare Technology Leadership by Frost & Sullivan in 2023 and 2025
Named among America’s Fastest-Growing Companies by Financial Times, 4 years in a row

Top Healthcare IT Developer and Advisor by Black Book™ survey 2023
Four-time finalist across HTN Awards programs

Named to The Healthcare Technology Report’s Top 25 Healthcare Software Companies of 2025

HIMSS Gold member advancing digital healthcare
ISO 13485-certified quality management system
ISO 27001-certified security management system
Typical Roles for HIPAA-Compliant Software Development
In most cases, ScienceSoft's HIPAA-compliant software development teams consist of:
Project manager
plans a healthcare software development project, assigns tasks to a project team, supervises the project delivery (including timing and budget), assesses project risks and provides solutions to mitigate them, and facilitates team communication and cooperation.
Business analyst
elicits medical software requirements and identifies tech limitations, creates the software concept and specification, analyzes healthcare software usage risks, designs software features, defines necessary app integrations with other software (e.g., EHR).
Solution architect
chooses a HIPAA-compliant technological stack, plans a healthcare software architecture taking into account compliance with HIPAA and other regulations.
Regulatory consultant for HIPAA compliance
advises on healthcare software architecture components, tech stack, development process, and project documentation management to ensure compliance with HIPAA and other relevant laws and standards.
UX designer
conducts UX research and identifies user scenarios, designs experiences of software users (patients, hospital supervisors, etc.) and interactions with software with a focus on usability and accessibility, creates UX prototypes.
UI designer
designs an appealing visual interface of healthcare software.
Back-end developer
creates the business logic and the server side of healthcare software with a focus on security and HIPAA compliance.
Front-end developer
develops the user side of healthcare software.
QA engineer
plans a test strategy, creates and executes test cases, reports medical software defects and vulnerabilities.
Information security specialist
creates security testing scenarios for HIPAA-compliant software and conducts security testing.
DevOps engineer
sets up and maintains medical software development infrastructure, establishes CI/CD pipelines for automated software deployment, selects and configures tools to execute daily monitoring of HIPAA-compliant software, etc.
Sourcing Models of HIPAA-Compliant Software Development
100% in-house HIPAA-compliant software development
- Full control over the medical software development project.
- High risks of HIPAA compliance and quality issues due to the lack of expertise with regulatory requirements, risk of project delays due to lack of resources and technical capabilities.
A mix of in-house and outsourced consultancy and development
- Access to necessary technical capabilities, highly qualified specialists in HIPAA-compliant software development, opportunity to scale up or down the resources when needed.
- Need for management of the outsourced resources and fast establishment of the communication process with the outsourced team.
Fully outsourced HIPAA-compliant software development process
- Full responsibility for healthcare project management, delivery, and HIPAA compliance lies on the vendor, minimal involvement on your side.
- High vendor dependency.
Benefits of HIPAA-Compliant Software Development Outsourcing with ScienceSoft
Healthcare industry regulations knowledge
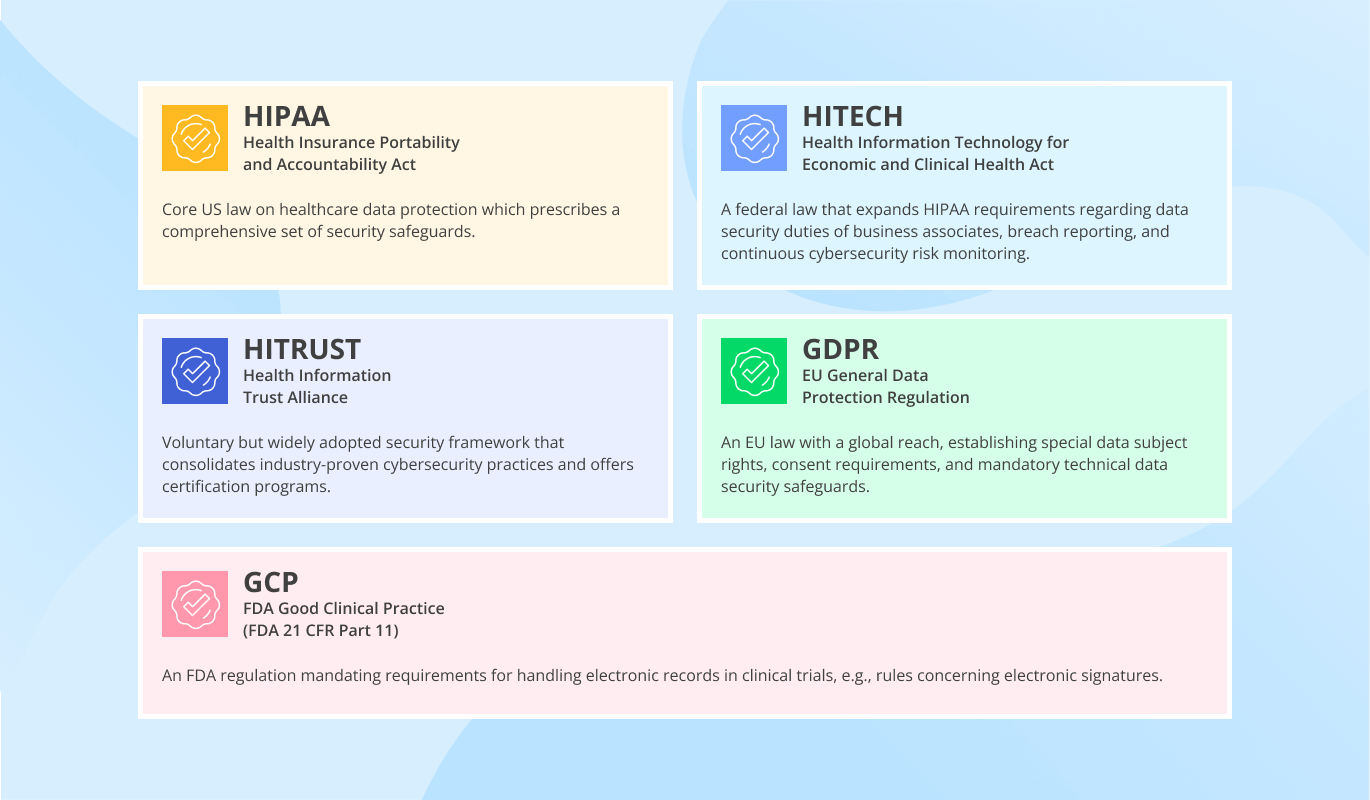
ScienceSoft plans and delivers medical software with a focus on relevant regulations (e.g., HIPAA, HITECH, Cures Act, FDA requirements), signs a Business Associate Agreement with the clients, and implements data security measures.
Fast solution delivery
ScienceSoft starts with HIPAA-compliant MVP development according to iterative approach to help you get ROI from medical software faster.
Optimized costs
To help you cut costs, ScienceSoft starts with a detailed requirements analysis to reduce risk of rework in the future, uses cloud-native architecture, and applies proven third-party components (e.g., for messaging).
Costs of HIPAA-Compliant Software Development
From ScienceSoft’s experience, each medical software project has specific tech requirements and limitations, so the cost factors vary from client to client. Here, we outline general cost factors for HIPAA-compliant software.
Core cost factors
- Medical software functionality scope (e.g., hospital asset tracking, patient rehabilitation).
- Number and complexity of features (e.g., IoT integrations will increase the cost).
- Healthcare software type (web, mobile, desktop).
- Supported mobile platforms (iOS, Android).
- Number of user roles (e.g., patients, doctors, administrators).
- Software scalability needs.
- Medical software performance requirements.
Integration cost factors
- Software integration with remote patient monitoring devices or tracking tags.
- Number and complexity of integrations with medical IT systems (EHR, practice management system, procurement software, etc.).
Operational cost factors
- Necessary data storage capacity.
- License fees for cloud services or ready-made components of HIPAA-compliant software (e.g., data analytics services, messaging services).
- Healthcare software maintenance services.
HIPAA-compliant software development costs range from $30,000 to $400,000+, depending on a solution's functionality. The price for a mobile patient app (e.g., for medication intake monitoring) without EHR integration starts from $30,000, telemedicine software – from $150,000 – $250,000, and a custom EHR system – from $400,000. These costs do not include regular license fees for cloud services.
Get a Cost Estimate for Your Healthcare Software Development Project
Please answer a few questions about your software development needs. This will help our team provide a tailored service offering and a cost estimate much quicker.
Thank you for your request!
We will analyze your case and get back to you within a business day to share a ballpark estimate.
In the meantime, would you like to learn more about ScienceSoft?
- Project success no matter what: learn how we make good on our mission.
- Since 2005 in healthcare IT services: check what we do.
- 4,200+ successful projects: explore our portfolio.
- 1,400+ incredible clients: read what they say.

Technologies ScienceSoft Uses for HIPAA-Compliant Software Development
ScienceSoft's healthcare IT team usually chooses the following tools and technologies during our HIPAA-compliant medical software projects:
HIPAA Compliance Checklist by ScienceSoft
We will be happy if you can check all the points below, as this would indicate that you are likely following all core HIPAA requirements.
|
|
About ScienceSoft
ScienceSoft is an IT consulting and software development vendor headquartered in McKinney, Texas, US. Being ISO 13485 certified, we design and develop medical software according to the requirements of the FDA and the Council of the European Union and ensure software compliance with HIPAA and HITECH regulations.