Healthcare Software Testing
Key Types, Plan, Cost Factors
Having 36 years of experience in software testing and 20 years in healthcare IT, ScienceSoft helps healthcare providers and medical software vendors ensure comprehensive testing of their healthcare solutions.
Healthcare Software Testing in Brief
Healthcare software testing helps ensure that medical IT solutions function as intended, comply with the required standards and regulations (FDA, HIPAA, etc.), use required standards for data exchange (HL7, DICOM, etc.), and guarantee security of patient information.
Testing costs may amount up to 40% of total software development costs, but outsourcing the testing to experts and applying test automation can help speed up the process and significantly reduce the healthcare testing expenses.
- Key steps: software requirements verification, risks evaluation, test planning and design, test execution, results reporting.
- Sample team structure: a QA manager, a test lead, test engineers, test automation engineers, a compliance consultant, an information security specialist.
Healthcare Software Testing Plan
To build a comprehensive healthcare software testing plan, you need to take into account the type of software under test (e.g., EHR/EMR, HIS, medical imaging and lab software, telemedicine solutions, SaMD, etc.), its complexity, required testing types, and the mandatory standards that the app must comply with (e.g., HIPAA in the US, GDPR in the EU). Specific plans may vary drastically depending on a solution’s functionality and chosen testing approach, but ScienceSoft’s experts outlined the main steps any medical software testing project goes through.
1.
Software requirement analysis and test planning
Depending on the complexity of the healthcare software and the project’s scope, a test lead or a QA manager:
- Analyzes the healthcare software requirements to verify that they are clear, complete, and testable.
- Defines the risks of a healthcare software testing project (e.g., shortage of resources, budget overruns, etc.) and creates a risk mitigation plan.
- Outlines the healthcare software testing plan that includes:
- Testing objectives and types of testing to perform.
- Testing schedule.
- Talents required & team(s) structure.
- Test automation share.
- Testing metrics.
If you are planning the development of a healthcare solution, I recommend involving QA specialists as early as at the requirements elicitation stage. QA experts will verify that the requirements are consistent and testable and, if needed, help redesign the requirements before the development process starts.
2.
Test design
After verifying the healthcare software requirements and defining the testing strategy, test engineers:
- Prepare relevant test environment and test data.
Best practice: During healthcare software testing, ScienceSoft uses mock data sets instead of real patient information to prevent the risk of sensitive data leakage.
- Design test scenarios and test cases.
- Prepare the testing toolkit (defects management & tracking software, communication tools, etc.).
- Set up and configure test automation tools and frameworks; write test automation scripts (where test automation is feasible).
To successfully register your medical device or SaMD with authorities such as FDA, you will need to submit technical documentation proving that the application is safe and works as intended. With that in mind, I strongly recommend documenting all testing activities in accordance with ISO/IEC 62304:2006 (Amendment 1:2015) and ISO 13485.
3.
Test execution and results reporting
The healthcare software testing engineers:
- Run and maintain manual test cases and automated scripts.
- Report on the found defects.
- Conduct re-testing and regression testing to validate that the defects are fixed and existing healthcare software functions are not affected.
- Submit regular reports on test execution and fulfillment of testing KPIs.
To measure the effectiveness of your healthcare software testing process, it’s best to use fulfillment KPIs such as:
- Requirements coverage.
- Average and total number of created, executed, and updated test cases during a defined interval of time.
- Ratio of reopened issues to resolved ones.
- Rejected defects.
- Defects leakage.
- Budget and time variance.
Key Healthcare Software Testing Types
To navigate you through possible testing types your project might need, we have summarized the types commonly requested by our clients in the healthcare domain.
The functionality of healthcare software is tested against the requirements to ensure that the healthcare solution works exactly as planned. Automation may be employed where feasible (e.g., regression testing) to significantly reduce the testing costs and testing time.
Testers check the stability of a healthcare solution under the normal load in stress conditions.
Security & compliance testing
For healthcare software, security and compliance testing are closely tied: ensuring the security of patient data is essential for compliance with standards and regulations like HIPAA and GDPR. Testing activities include penetration testing, vulnerability assessment, security code review, etc.
Compatibility testing
Test engineers conduct compatibility testing to ensure that a healthcare application can run on different devices and is fully compatible with the required range of operating systems and browsers.
Interoperability testing
Test engineers check if a healthcare solution is able to reliably exchange medical data in accordance with major data transfer standards such as HL7, FHIR, DICOM, and more.
Testers check whether combined healthcare software modules or healthcare applications (e.g., EHR, RCM, patient apps, medical devices, imaging and lab software, etc.) work together smoothly.
With regard to the healthcare industry rules and regulations, test engineers develop multiple user scenarios for each user role (e.g., various medical staff, admins, patients, etc.) to cover every procedure a specific user performs and to make sure the solution offers smooth and convenient user experience.
Consider Professional Services for Healthcare Software Testing
ScienceSoft leverages its 36 years of experience in software testing and 20 years in healthcare IT to help our clients ensure that their healthcare application is fully functional, stable, secure, and compliant with the required standards and regulations.
Healthcare software testing consulting
ScienceSoft’s consultants will:
- Analyze your healthcare solution’s requirements and specifics.
- Offer an optimal sourcing model based on your testing needs, healthcare application specifics, and budget.
- Design a detailed healthcare software testing plan tailored to your healthcare solution’s specifics.
- Help select optimal testing frameworks and tools.
- Develop a set of KPIs for the healthcare software testing project.
- Identify healthcare software testing risks and create a risk mitigation plan.
- Provide healthcare software costs breakdown and advise on possible costs optimization strategies.
Healthcare software testing outsourcing
ScienceSoft’s testing experts will:
- Design a healthcare application testing strategy and plan, choose the appropriate testing types, frameworks, and tools.
- Set up the test environment and healthcare test data generation process.
- Develop, execute, and maintain healthcare test cases and test automation scripts.
- Introduce test automation (if required).
- Provide comprehensive documentation of the testing activities.
- Test healthcare software for compliance with the required regulations and standards.
- Regularly report on the progress of healthcare software testing and offer optimization opportunities.
ScienceSoft as a Healthcare Software Testing Company
- 36 years in software testing.
- 20 years in healthcare IT.
- ISTQB-certified testing engineers.
- Quality management system for medical device software and SaMD proven by ISO 13485 certification.
- ISO 27001 and ISO 9001-certified high-quality testing services and the security of sensitive data.
- Working experience with major healthcare regulations (HIPAA, HITECH, ONC, MACRA, MIPS, CEHRT, SAFER) and healthcare data exchange standards (e.g., HL7, ICD-10, CPT, XDS/XDS-I).
Our awards, recognitions, and certifications
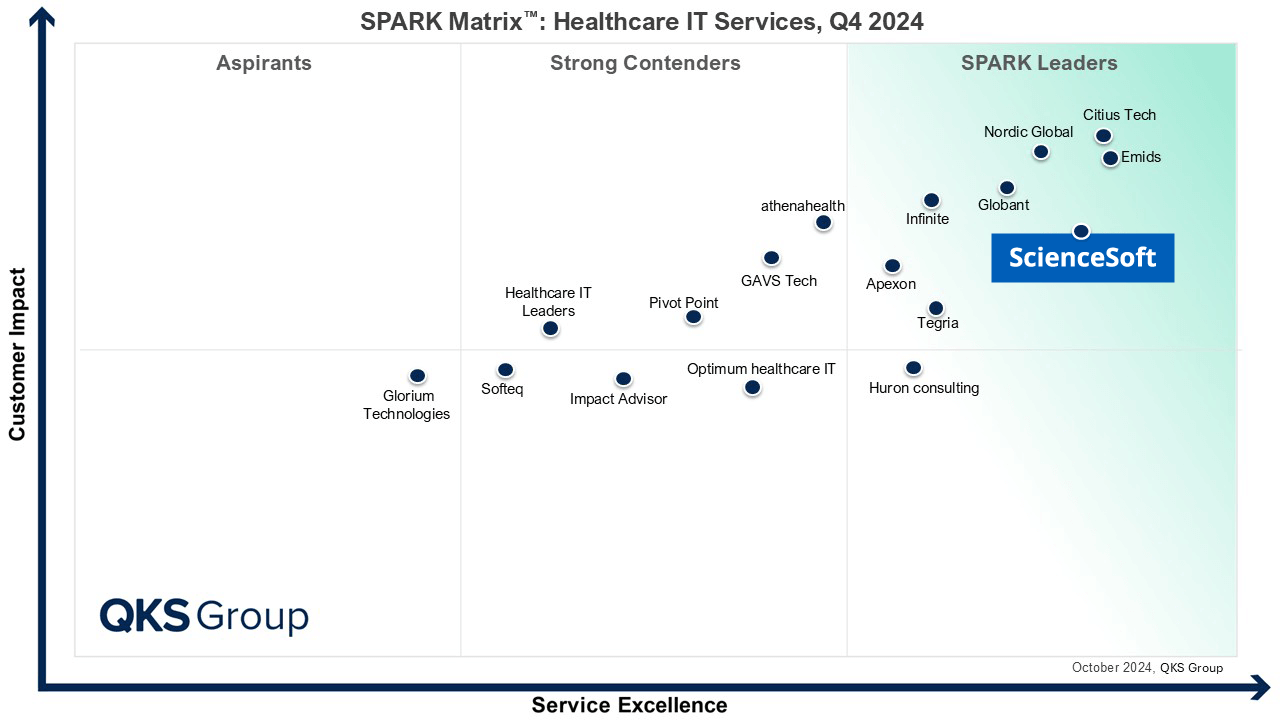
Featured among Healthcare IT Services Leaders in the 2022 and 2024 SPARK Matrix
Recognized for Healthcare Technology Leadership by Frost & Sullivan in 2023 and 2025
Named among America’s Fastest-Growing Companies by Financial Times, 4 years in a row

Top Healthcare IT Developer and Advisor by Black Book™ survey 2023
Recognized by Health Tech Newspaper awards for the third time (2022, 2023, 2025)

Named to The Healthcare Technology Report’s Top 25 Healthcare Software Companies of 2025

HIMSS Gold member advancing digital healthcare
ISO 13485-certified quality management system
ISO 27001-certified security management system
Typical Roles on ScienceSoft's Healthcare Software Testing Team
The specific team composition for a healthcare application testing project will strongly depend on the type and complexity of medical software, as well as the required testing activities. Below are the most common roles in ScienceSoft’s healthcare software testing teams.
QA manager (for projects with several testing teams)
- Helps design comprehensive and testable healthcare application requirements.
- Identifies policies and regulations that define the compliance needs of a healthcare solution.
- Develops a healthcare software testing strategy and chooses test management tools.
- Gathers the required talents and testing teams.
- Guides the setup of the QA process.
- Tracks and improves testing KPIs.
Test lead
- Designs a test plan according to the type and complexity of a healthcare solution.
- Defines the testing frameworks and tools relevant for the healthcare solution under test.
- Manages the testing team members.
- Controls test design and test execution.
- Ensures that the healthcare software testing goals are met and delivered on time.
Test engineer
- Designs and maintains test cases.
- Conducts manual healthcare application testing.
- Reports on the defects found and validates the fixed defects.
Test automation engineer
- Defines which test scenarios should be automated.
- Designs test automation architecture.
- Selects and configures test automation tools.
- Develops, executes, and maintains test automation scripts.
- Reports on the defects found and validates the fixed defects.
Compliance consultant
- Reviews the software requirements, testing process, tools, and project documentation to ensure compliance with the applicable healthcare standards and regulations.
- Cooperates with the testing team to ensure secure handling of sensitive data as required by regulations (e.g., HIPAA, GDPR) during the testing process.
Cybersecurity specialist
- Conducts vulnerability assessment and penetration testing to identify potential security loopholes.
- Advises on the ways to mitigate the revealed security issues, prevent potential data breaches, and reinforce the existing security controls.
Sourcing Models for Healthcare Software Testing
In-house healthcare software testing
- Full control over the healthcare solution testing process.
- Potential lack of in-house talents with required testing skills or domain competences.
- High expenses on the staff’s training, salaries, taxes, licensed testing tools, etc.
- Low scalability of the team.
Outsourced healthcare software testing
- Optimized healthcare software testing costs.
- Scalable teams of testing experts experienced in healthcare industry.
- Transparent KPI-based approach to testing.
- Risks related to vendor choice.
QA management is in-house; a testing team is completely or partially outsourced
- Balanced costs and control over the testing process.
- Quick access to talents with required skills or experience with a specific healthcare solution type, standard, etc.
- Possible communication issues between the in-house management and outsourced team.
Software ScienceSoft Applies in Projects
Automated UI testing tools
Automated mobile testing tools
API testing
Performance testing tools
Security testing tools
CI/CD tools
Test management and defect tracking software
Factors Influencing Healthcare Software Testing Costs
Healthcare solution-related factors:
- The type of a healthcare solution and its complexity.
- Specific security and compliance requirements (e.g., HIPAA, GDPR).
- The number of user roles (e.g., medical staff, admins, patients, etc.) and the intended number of users.
- The number and complexity of integrations with other healthcare IT systems (EHR, RCM, CRM, practice management system, procurement software, patient apps, etc.).
Cost factors related to testing process:
For an in-house option
- The QA teams’ hiring costs and full salary expenses.
- The costs of additional training on the healthcare application testing for the in-house team (if required).
- The costs of employed tools and test environment (e.g., licenses for the chosen testing tools and frameworks, storage, virtual machines).
For an outsourced option
- The QA professionals’ hourly rates (based on their competence and experience).
- The healthcare application testing time (based on the number and complexity of test cases, as well as development and maintenance efforts per test case).
- Test automation share (if applicable).

About ScienceSoft
ScienceSoft is a global IT consulting, software development and QA company headquartered in McKinney, TX. In the healthcare IT domain since 2005, ScienceSoft tests healthcare solutions of any scale and complexity, including software with advanced techs (IoT, AR, VR, ML, Big Data, etc.). Being ISO 13485, ISO 9001 and ISO 27001-certified, ScienceSoft guarantees high-quality testing of healthcare software and ensures the security of sensitive data. If you are interested in testing your healthcare solution, contact our professional healthcare application testing team.

