Interactive Response Technology (IRT) Software for Clinical Trials
In healthcare IT since 2005, ScienceSoft engineers IRT solutions for CROs, pharmaceutical, and biotech companies. We deliver IRT software that can be easily aligned with unique clinical trial protocols and regulations. Our custom solutions feature automated workflows to minimize manual efforts and advanced analytics to reduce IP wastage and shipment costs.
Interactive response technology (IRT) software automates participant randomization according to clinical trial protocol requirements, ensures compliant investigational product (IP) shipment and dispensing, keeps accountability records, and prevents unblinding throughout the trial.
The term "interactive response technology" gets its meaning from its early use via telephone systems. Today, it's also commonly known as a randomization and trial supply management (RTSM) system.
Research organizations' key demand for an IRT system is the ability to adapt rapidly to protocol requirements. In a recent IRT trends survey involving 100 R&D companies, respondents reported that the complexity of study start-up and protocol amendment implementation (62%) and a lack of technical flexibility (41%) were the main drawbacks of their IRT software that caused trial delays.
Custom interactive response technology (IRT) is chosen by research organizations when the functionality of off-the-shelf solutions does not allow them to:
- Fulfill the demands of complex trials, such as research involving multiple stratification factors, dose titrations, and adaptive designs.
- Manage supply chain across multiple countries and sites, effectively collaborate with international shipping companies, and adhere to all local regulations.
- Analyze real-time data on recruitment and IP inventory to enable prompt decisions on extra manufacturing or supply.
Implementation time: from 8 to 16+ months.
Useful integrations for IRT software: eConsent software, electronic clinical outcome assessment (eCOA) software, electronic data capture (EDC) software, a clinical trial management system (CTMS), delivery service providers’ systems, and more.
Costs: $130,000–$800,000+, depending on software complexity. Use our free calculator to get an approximate cost estimate for your project.
Essential Features of IRT Solutions
ScienceSoft’s healthcare consultants highlight these features as most requested by our clients from the clinical R&D sector. In real-life projects, when designing IRT for clinical trials, our business analysts carefully select the optimal feature set to meet the needs of each specific research organization.
Essential Systems to Integrate With IRT Software
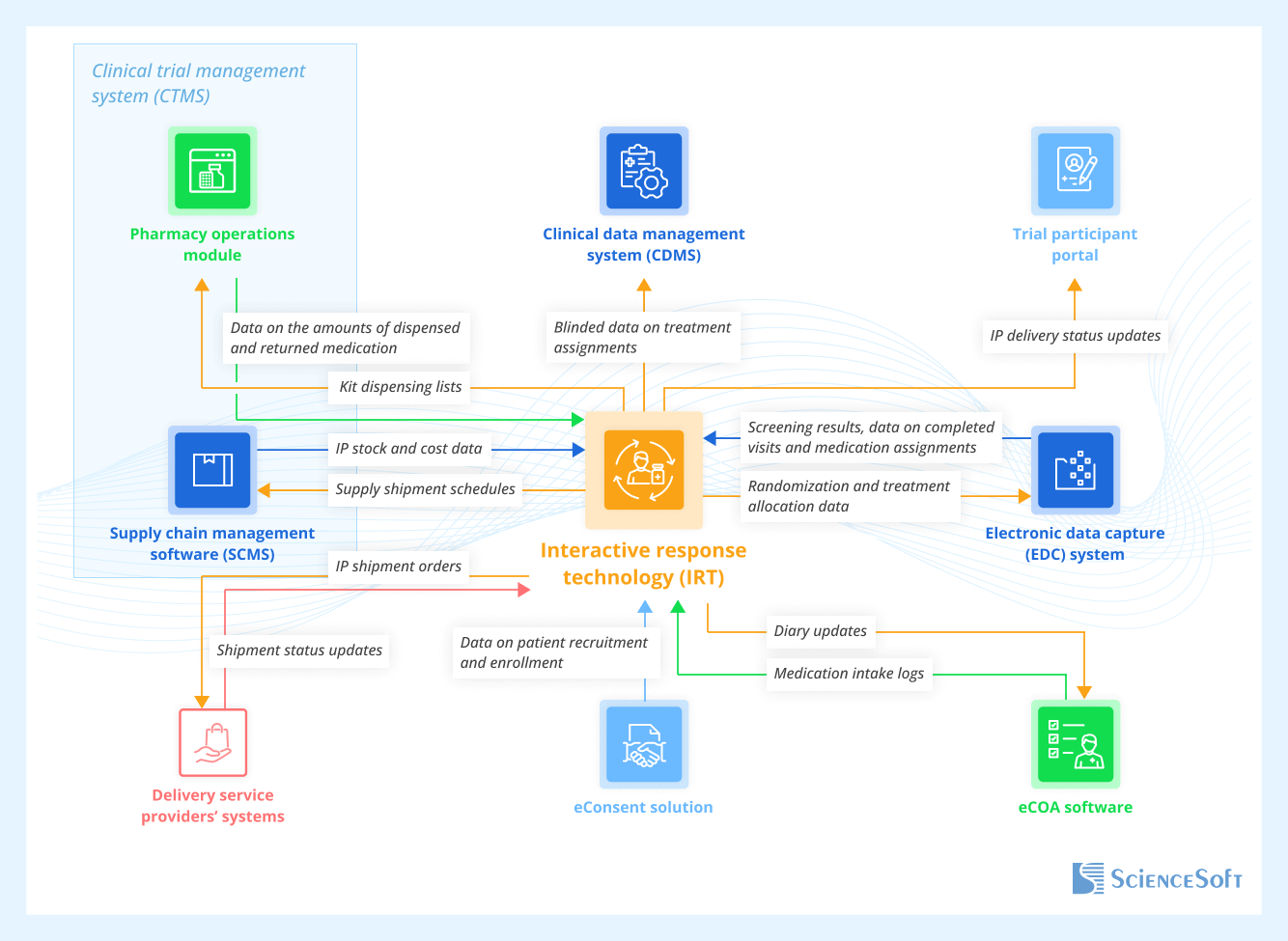
- An eConsent solution — to obtain data on patients who have been recruited, undergone screening, and provided informed consent for the study.
- Electronic clinical outcome assessment (eCOA) software — to collect information on the amount of consumed medication from patient diaries; to update diaries according to the patient’s current trial phase (screening, dose titration, hospital treatment, planned follow-up, etc.).
- An electronic data capture (EDC) system — to obtain screening results for assessing patient eligibility (often using ICD-10 and SNOMED CT codes); to track patient visits, treatment timelines, unscheduled visits, and other events essential for IP supply planning.
- A pharmacy operations module of a clinical trial management system (CTMS) — to enable automatic kit matching with the right patients; to collect information about the medication dispensed and returned by patients.
- Supply chain management software (SCMS) — to provide sites with delivery schedules and enable real-time IP stock tracking; to get data on IP and shipment costs for reporting expenses and improving site supply cost efficiency.
- Delivery service providers’ systems — to automatically place shipment orders and track IP delivery in real time.
- A clinical data management system (CDMS) — to feed IRT-captured randomized treatment details into a centralized trial data repository via clinical data integration pipelines.
Main Steps of Building Custom IRT Software
Below, ScienceSoft’s project managers share the key steps and best practices needed to deliver flexible, compliant, and efficient interactive response technology (IRT) software.
1.
Business analysis and defining software requirements
IT consultants work with representatives from clinical trial sponsors and CROs, including managers, trial coordinators, investigators, and site staff. Their goal is to determine the optimal feature set that can meet the research organization’s current and potential future needs.
For example, for a sponsor using IRT in clinical trials of an expensive chemotherapy drug with a limited shelf life, the main goal could be minimizing the drug's wastage. To accomplish this, the system may require advanced AI-powered supply optimization to prevent excess IP production and coordinate delivery straight from the production unit to the patient’s department shortly before the infusion is due.
If the research involves a complex study design and a large number of stratification factors, features for automatic randomization balance control may be needed. This may include real-time monitoring of the balance between patient strata, alerting researchers in case of any imbalance, and suggesting corrective actions, such as halting the recruitment of patients with certain characteristics.
2.
Architecture design
Software architects carefully analyze the software requirements to design the optimal architecture for the future solution.
For interactive response technology in clinical trials, ensuring the solution's flexibility is crucial, as research organizations need to swiftly adapt the system to mid-study changes, such as protocol amendments. To accomplish this, architects often design a multi-tier architecture with a dedicated configuration component or rule engine.
This approach allows trial managers to modify the number of cohorts or visit schedules directly through the IRT interface without having to alter the software code. These changes are applied at the configuration level and propagate to all relevant workflows without causing inconsistencies. Separating the business logic layer ensures that core functionalities (e.g., randomization algorithms and drug assignment rules) continue to operate correctly despite any modifications.
At the same time, architects ensure the system is ready for standardized interoperability by supporting FHIR APIs and handling patient data sets in line with USCDI requirements.
In IRT projects, when clients need the ability to quickly adapt the software to protocol amendments, we often turn to low-code technologies. With low-code platforms, such as Microsoft Power Apps, we provide trial managers with a drag-and-drop workflow editor that lets them easily adjust randomization and IP supply rules, saving costs and preventing delays in the research process.
3.
UX and UI design
UX designers plan user journeys and interfaces for different trial roles to minimize the number of clicks required to complete common tasks, thereby enhancing data entry accuracy and reducing task completion time.
For example, to make clinical trial pharmacists' work easier, the system can auto-populate fields based on previously entered data, such as patient IDs or dispensed kit details. Critical actions, such as dispensing or recording medication returns, can be prominently displayed in the front and center of the pharmacist’s workspace and designed to require the fewest number of steps to complete.
Furthermore, when registering returned empty packages and unused medication, the system may alert a pharmacist if the data does not match the amount of medications recorded as consumed in the patient's diary. Such alerts should be designed with prominent visual cues, such as color-coded notifications or pop-up messages, to immediately capture the pharmacist’s attention.
To ensure that clinicians and pharmacists see consistent terminology across different trial systems, the interface can be aligned with medical coding standards such as SNOMED CT, LOINC, and RxNorm.
4.
Iterative development and testing
ScienceSoft usually recommends an iterative development approach to test features as they’re completed and catch issues as early as possible. Once each significant development milestone is achieved, engineers show the finished functionality to future users and ask them to provide feedback so that the team can make any necessary adjustments. For example, developers may ask researchers to generate test randomization lists and check that the workflow configuring the randomization scheme completely fits their needs.
As for the quality assurance in IRT projects, compliance and security testing get particular attention. For the system to comply with HIPAA, GDPR, FDA 21 CFR Part 11, and other relevant regulations, testers have to verify that access to trial data is limited according to the principle of least privilege (PoLP), that any data access or changes are thoroughly logged, and that controls for data completeness and integrity work properly.
It’s also important to verify the safeguards against accidental unblinding. For example, this means that IRT business logic should prevent situations where sites receive IP kits with consecutive numbers, which may indicate that they are intended for patients belonging to the same group.
5.
Deployment and support
After the IRT system goes live, the development team supports the research staff by providing user manuals and offering consultations. They also monitor system performance and resolve any remaining issues. For large-scale international studies, a dedicated support team and a 24/7 help desk are typically required.
ScienceSoft usually offers free software maintenance and support for the first few months after software delivery (the exact duration is dictated by the chosen post-launch warranty terms). During this time, we can either gradually transfer knowledge to your in-house IT team or assemble a team of our support agents to handle continuous software maintenance, evolution, and L1–L3 help desk.
How Much Does It Cost to Develop Custom IRT Software?
Based on ScienceSoft’s experience, the development cost for custom interactive response technology software ranges from $130,000 to over $800,000. The most significant factors affecting the cost are the need to support complex study designs, the number of software integrations, the need for advanced supply management automation, and AI capabilities.
Below is a rough cost estimate for a custom IRT system for clinical trials with basic, standard, and advanced capabilities.
From $130,000
A basic solution with the following essential capabilities:
- Support for common study randomization schemas.
- Automated IP inventory control and resupply scheduling.
- IP expiry management.
- Basic analytics and reporting for patient enrollment and IP inventory.
- Integrations with EDC and CTMS software.
From $300,000
A standard solution with the following extra features beyond the basic capabilities:
- Support for complex trial designs, dosage regimens, and resupply strategies.
- Automated shipment ordering and tracking.
- Automated IP accountability records.
- Real-time supply chain analytics.
- Integration with up to 5 internal systems.
From $800,000
An advanced solution with the following extra features beyond the standard capabilities:
- Computer vision capabilities for automatically identifying kits and counting returned medication.
- ML/AI-assisted supply chain optimization and out-of-stock risk management.
- Integration with up to 10 systems, including external delivery service providers.
Why Choose ScienceSoft for Your IRT Project
- Since 2005 in healthcare IT.
- 150+ successful projects in the domain.
- Experience in meeting GCP, FDA, HIPAA, HITECH, GDPR, and Cures Act requirements.
- Since 1989 in data analytics, data science, and AI.
- Niche experts, including project managers, principal architects, healthcare IT consultants, and engineers with expertise in clinical research software development.
Our awards, recognitions, and certifications
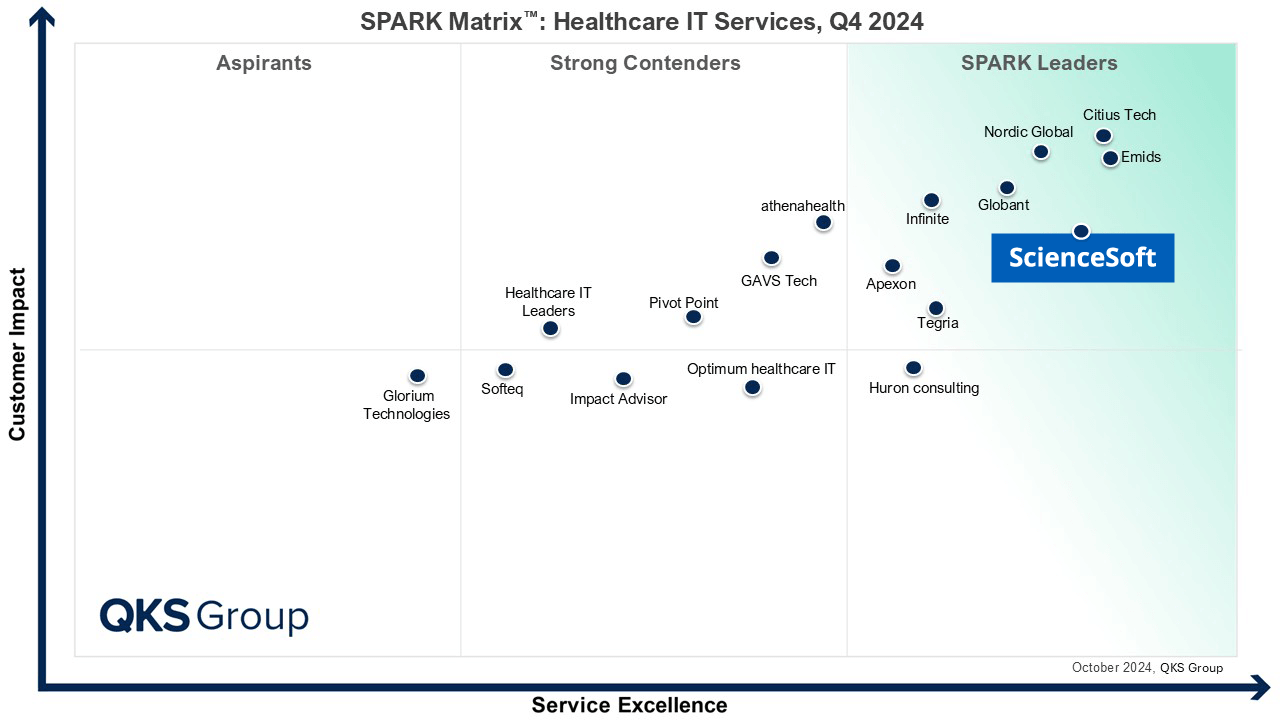
Featured among Healthcare IT Services Leaders in the 2022 and 2024 SPARK Matrix
Recognized for Healthcare Technology Leadership by Frost & Sullivan in 2023 and 2025
Named among America’s Fastest-Growing Companies by Financial Times, 4 years in a row

Top Healthcare IT Developer and Advisor by Black Book™ survey 2023
Four-time finalist across HTN Awards programs

Named to The Healthcare Technology Report’s Top 25 Healthcare Software Companies of 2025

HIMSS Gold member advancing digital healthcare
ISO 13485-certified quality management system
ISO 27001-certified security management system




