Quantitative imaging biomarkers: From subjective observations to objective characteristics
Conventional qualitative and subjective methods of medical image interpretation are ill-reputed due to their observer-dependent and highly variable nature, which may affect diagnostic accuracy and patient outcomes. To reduce variability and sustain higher patient outcomes, objective quantitative imaging biomarkers (QIBs) are being introduced into medical image analysis.
Quantitative imaging biomarkers are objective physical and biological characteristics calculated and measured from any type of medical image. QIBs can help identify normal biological processes, pathogenic processes or response to treatment and intervention.
3 applications of quantitative imaging biomarkers in healthcare
Connecting medical images to biological events
QIBs show how the disease affects a patient’s organs and systems. Such details can be crucial for making an accurate diagnosis, defining the precise treatment plan, and evaluating the treatment response.
Let’s take the example of nonalcoholic steatohepatitis. This condition involves liver steatosis and inflammation, leading to chronic liver disease and cirrhosis. It is necessary to measure liver steatosis and inflammation together, as both QIBs are synergistic toward the parenchymal fibrosis progression.
In this case, MRI images in multiple sequences allow for the extraction of the needed quantitative biomarkers. T1-T2 sequences can help estimate the fat deposit, and a diffusion-weighted MRI image with the intravoxel incoherent motion model gives an estimation of the inflammation state. In combination, these sequences create a multivariate prognostic biomarker for early diagnosis and grading of steatohepatitis.
Supporting precision medicine and decision-making at the bedside
Imaging biomarkers, comparable to specimen biomarkers, provide information about molecular processes in a patient’s body. However, specimen biomarkers have a few limitations, which imaging biomarkers can overcome.
Biological specimens are usually acquired via biopsy or from body fluids. The main disadvantage is that specimen collecting presumes taking only a small part of the normal or damaged tissue, which causes spatial sampling bias. Imaging scans, in their turn, usually show either a broad tissue segment or even the entire patient, therefore allowing for more comprehensive heterogeneous data.
Besides, imaging data can be acquired dynamically and longitudinally without additional invasive procedures, which is impossible for specimen biomarkers when the frequent and continuous analysis of tissue or fluid is needed.
Accordingly, imaging biomarkers can help providers to close the gaps related to specimen biomarkers and improve therapeutic development, treatment monitoring, and overall decision-making at the bedside. Moreover, current computing capacities and image processing methods are capable of extracting QIBs at little or no increased cost, which facilitates the integration of quantitative imaging into the radiology department workflows.
Rethinking medical imaging technology
According to MedicalPhysicsWeb, with the imaging technology advances of the past few years, we are getting closer to the practical limits of spatial resolution for almost all modalities. Therefore, the focus of improving imaging technology gradually shifts from image quality to image information value.
While a medical image allows for recognizing, measuring, and mapping visible changes in tissues, blood flow, bones, and other structures within a patient’s body, there is still a gap between imaging measurements and underlying conditions. For example, the functional brain architecture can be easily studied via functional MRI, yet connecting the map of neural activity and the underlying neurochemical and electrophysiological processes is still problematic.
Quantitative imaging biomarkers can reflect specific physiological phenomena or biophysical properties, providing needed image-condition connections. Developing them further is the next stage in the evolution of imaging technology, changing the medical imaging objective from showing the ‘best possible’ picture to extracting valuable insights.
Quantitative imaging biomarkers across modalities
As the current medical imaging technology provides a high-resolution view of a patient’s body, it is possible to extract various quantitative imaging biomarkers within most modalities:
- A CT signal has a high spatial resolution; therefore, CT scans can provide accurate distance measurements, including: basic tissue characterization, tumor dimensions or volume, quantitative physiologic information related to perfusion or necrosis, angiographic information, and functional information using dynamic contrast techniques.
- With appropriate calibration and standardization, an MRI signal response can be translated into quantitative biomarkers of tissue structure and function.
- A PET signal has high sensitivity. PET radiopharmaceuticals are able to assay a wide variety of physiologic or molecular functions.
- Ultrasound signals are a good fit for QIBs. They allow for the extraction of quantitative information about reflection, attenuation, refraction, bulk tissue properties, and shear wave speed. Additionally, quantitative distance, elasticity, and Doppler flow measurements can be calculated.
2 models of imaging biomarkers
There are two main models of imaging biomarkers: the static anatomic model and the biological dynamic model.
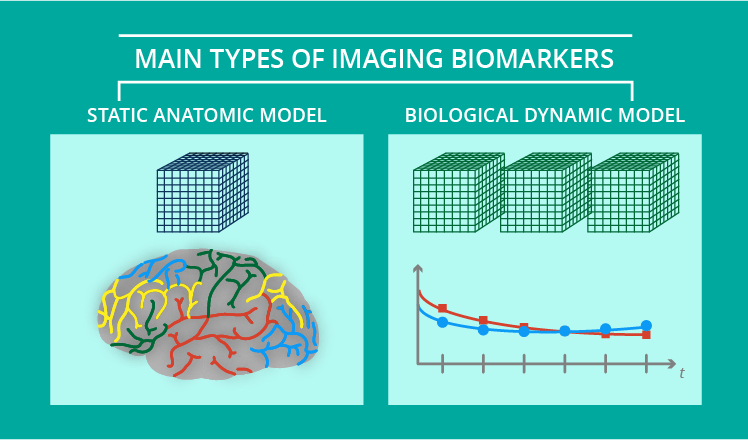
The static anatomical model estimates tissue aspects related to the volume and shape of the tissues, topology, and co-occurrence matrix features for texture classification. For example, cortical thickness and lung emphysema analyses are considered static methods.
The dynamic biological model assesses different physical, chemical, and biological hallmarks, such as fat and iron measurements within the pancreas. These biomarkers are obtained after biological dynamic modeling of the acquired data.
Challenges of implementing QIBs
The major challenge that hinders widespread adoption of QIBs is rooted in the complexity of QIBs' development and implementation processes. These processes have several consecutive steps, including the definition of the target hallmark, source images, analytical methodology, and type of measurements.
To validate and integrate quantitative imaging biomarkers into clinical practice, they need to comply with such requirements as conceptual consistency, technical reproducibility, adequate accuracy, and meaningful appropriateness. Ensuring such a level of standardization in QIBs requires coordinated effort between multiple parties: imaging device and software manufacturers, regulatory organizations, providers, academic institutions, and more.
Additionally, the continuous technological advancement in medical imaging hardware and software requires constant reassessment of the quantitative accuracy of medical images and regular updates of standardization requirements.
The solution for implementation challenges
To drive development, validation, and integration of QIBs, the Radiological Society of North America (RSNA) organized the Quantitative Imaging Biomarkers Alliance (QIBA). This collaboration’s mission is to improve the value and practicality of quantitative imaging biomarkers by reducing variability across devices, patients, and time.
To achieve that, they came up with the so-called QIBA Profile. This document describes in detail a quantitative imaging biomarker to be validated, explaining such properties as:
- QIB intended use or clinical context
- An image acquisition protocol
- Compliance items
- A claim of the achievable minimum variability/bias
Depending on the QIBs described, each Profile is designed for varying audiences, including imaging device manufacturers, pharmaceutical companies, researchers, technologists, physicians, accreditation and regulatory authorities, and more.
Distant prospect of QIBs adoption
From what we are seeing now, the medical imaging technology evolves in a direction that is beneficial for the development of quantitative imaging biomarkers. However, there are multiple challenges on the way to their real-life integration.
Apart from the fact that the implementation of QIBs can be hindered by the “inconsistent or incorrect use of terminology or methods for technical performance and statistical concepts,” according to QIBA, there are infrastructure-related issues as well.
To enable the use of QIBs in clinical and research settings, a whole network of developed, standardized, and optimized imaging acquisition protocols, display methods, image analysis guidelines, and reporting structures needs to emerge. It will take significant time, even with QIBA being on board, to facilitate major challenges.
Still, with a high potential of quantitative imaging biomarkers for precision medicine, diagnostics, and drug research, this effort will eventually be rewarding.


